Question
Question: How can I know how many hydrogens are there for each carbon in bond line notations?...
How can I know how many hydrogens are there for each carbon in bond line notations?
Solution
Carbon has four valence electrons in its outer shell and carbon is going to form four bonds with other atoms. Hydrogen has one valence electron and hydrogen is going to form a single bond with other atoms.
Complete step by step answer:
- In the question it is asked how we can know how many hydrogens are there on each carbon in a bond line notation.
- Bond line notation is a simple way to represent the simple organic compounds.
- In bond line notation there is involvement of carbon and hydrogen representation in the organic molecule and it can be represented by taking an example of propane and it is as follows.
- Now coming to the main concept we can find how many hydrogens are there on each carbon atom in bond line notation.
- We know that carbon is going to form four bonds with other atoms.
- In bond line notation first we have to calculate how bonds are there with one carbon.
- For example take propane :
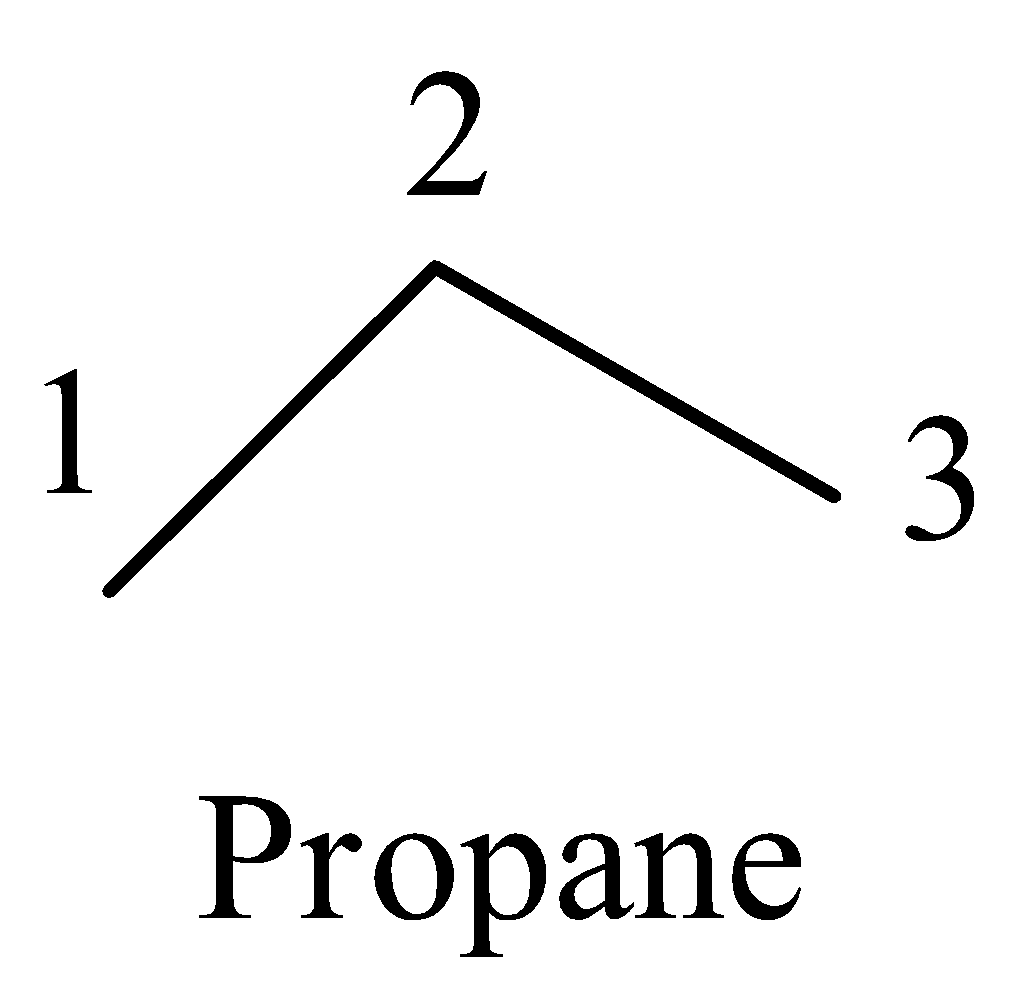
- The carbon one has one bond with carbon two means the remaining three valence electrons of carbon are going to form three bonds with three hydrogen atoms. Means on carbon one there are three hydrogens.
- Coming to carbon-2, it is bonded to carbon-1 and carbon-3, then the remaining two valence electrons are going to form bonds with two more hydrogens than the carbon-2 has two hydrogens on it.
- This is how we can calculate the number of hydrogens on carbon in bond line notation.
Note: It is very easy to find the number of hydrogens on the carbon in bond line notation, just we are supposed to do it if we have to find that the carbon atom has how many bonds with the other carbon atoms in the given molecule.
