Question
Question: How can I identify and draw the optical isomers for the isomers of : \[{[Cr{({H_2}O)_3}C{l_3}]^ + }\...
How can I identify and draw the optical isomers for the isomers of : [Cr(H2O)3Cl3]+
Solution
The given compound is a coordination complex formed by the chromium metal. The complex contains a total of six ligands around the central metal ion. Three out of six ligands are water molecules which are neutral and the remaining three are chloride ions.
Complete answer:
Metals belonging to the d-block of the modern periodic table tend to form coordination complexes due to their ability to show variable oxidation states and high oxidation states that allow them to form bonds with multiple groups. These groups that provide electrons to the electron deficient central atom are called ligands.
Ligands can be neutral, negatively charged or positively charged and all of them form coordinate covalent bonds with d-block elements where they fill up the vacant d-orbitals of metal with their electron pairs.
Optical isomerism is observed in such complexes in which the formula and structure of the complexes remains the same but their spatial arrangement varies due to which they can become optically active or inactive.
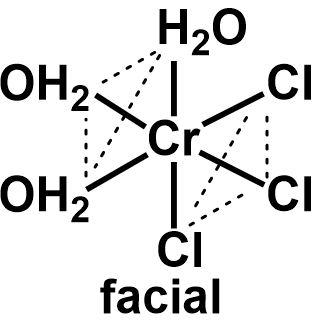
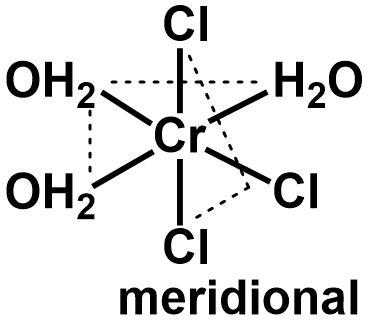
The given complex [Cr(H2O)3Cl3]+ is a chromium complex with three water molecules and three chloride ions as ligands. Since there are six ligands, it is an octahedral complex with half of the ligands of one type and the other half of another type. Such a complex is categorized as a MX3Y3 complex that shows facial and meridional isomerism.
The facial and meridional isomers are types of optical isomers observed in octahedral complexes only. When the three ligands of one type occupy one face of the octahedron and the other three ligands occupy the other three faces, it is called a facial isomer. If the identical ligands are arranged in a manner that two of them are cis to each and another one is trans, then it is called a meridional isomer.
⇒ The two isomers can be drawn as follows:
Note:
Cis ligands in an octahedral geometry are placed perpendicular to each other and the trans ligands are placed in a linear fashion where they occupy anti parallel positions to one another. All the identical ligands in a facial isomer are cis to each other.
