Question
Question: How can I draw two equivalent resonance structures for the formate ion, \({\text{HCO}}_{\text{2}}^{\...
How can I draw two equivalent resonance structures for the formate ion, HCO2 - ?
Solution
Hint For the formation of equivalent resonance structures of formate ion (HCO2 - ), first we have to know about the valence shell or outermost electrons of each atoms present in the molecule.
Complete step by step solution:
Steps which are required for the formation of equivalent resonance structures for the formate ion are as follow:
-In the former ion, carbon atom is the central atom because it is more electropositive than oxygen atom.
-Now we calculate total number of valence electrons of HCO2 - by adding valence electrons of all atoms and charge present in it.
-Valence electrons in HCO2 - = 1+4+(2×6)+1=18.
-Now we form single bonds between each atom and carbon atom.
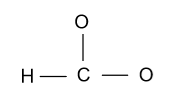
-After forming single bonds out of 18e - , only 12e - are left as six electrons are involved in the three single bonds.
-Now we divide this 12e - among two oxygen atoms then each oxygen atom will have three lone pair of electrons.
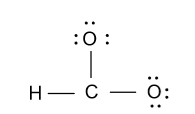
-As we know that a carbon atom bears four valence electrons but in the above diagram only three bonds are formed by the carbon atom. So we will convert one lone pair of electron from one oxygen atom to the double bond and we get following structure:
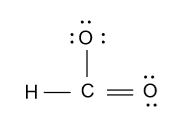
-In the above diagram we also have to show charge because number of electrons in the above diagram is counted by considering the negative charge’s electron.
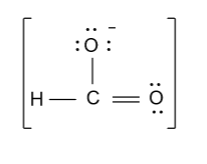
-Now the resonance structure of formate ion (HCO2 - ) are shown as follow:
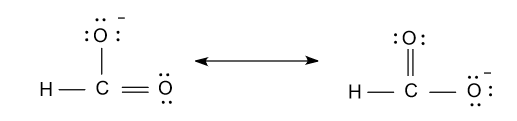
Note: Here some of you may think that why we didn’t divide 12e - among hydrogen atom also so, the reason is that in hydrogen only one valence electron is present and that value was fulfilled by the single bond. That’s why we divide 12e - among two oxygen atoms only.
