Question
Question: How can I draw the structures of the two enantiomers of cysteine?...
How can I draw the structures of the two enantiomers of cysteine?
Solution
Cysteine is a non-essential amino acid it produces in the human body. In order to draw the enantiomers of cysteine, we must draw them in fischer form and after that convert it into wedge-dash form. It is considered as a protein building block.
Complete step by step answer:
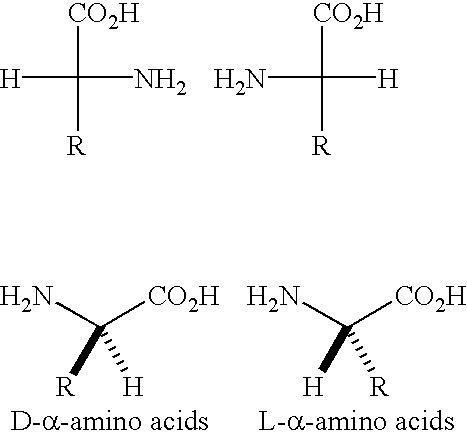
The most common way for drawing enantiomers is the Fischer projections.
The general formula for an amino acid is RCH(NH2)COOH . In cysteine, R=CH2SH .
The Fischer projection structure for cysteine is the same as the Fischer projection structure for a carbohydrate.
The main chain is vertical, with C−1 at the top. If the NH2 group is on the left, you have an L amino acid. If the NH2 group is on the right, you have an R amino acid.
After drawing fischer projection, we can easily convert it into wedge-dash formula.
If you draw the NH2−C−COOH bonds in the plane of the paper, with NH2 on the left and the pointing down, the L-amino acid has the bond to the R group as a dashed line.
If the points up, the L-amino acid has the bond to the R group as a wedge.
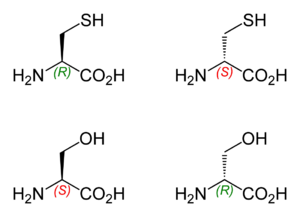
So the two structures of cysteine are those above, with R=CH2SH .
Note that L−cysteine has the R configuration. And L−serine has the S configuration.
Note:
An enantiomer is defined as the one of two stereoisomers which are mirror images of each other that are non-superposable. For example the left and right hands of a person are mirror images of each other which are non-superimposable and that cannot appear identical simply by reorientation.
