Question
Question: How can I draw the structural formulas for all the isomers of \({{C}_{4}}{{H}_{7}}Cl\)? Are there an...
How can I draw the structural formulas for all the isomers of C4H7Cl? Are there any enantiomers or diastereomers?
Solution
Isomers of a compound are the different structures that can be made from the empirical formula of the compound. Enantiomers and diastereomers, both contain a chiral centre.
Complete answer:
We are given the empirical formula C4H7Cl of a compound and to draw its possible structural isomers. For drawing the isomers of C4H7Cl, first we need to see the total carbons in this compound and the presence of double bonds.
When we remove Cl from C4H7Cl and add hydrogen, we get C4H8. But alkane with 4-carbons (butane) has a formula C4H10, this shows that a double bond is present in the compound C4H7Cl.
From the formula of C4H8, which is butene, we can draw :
-6 isomers of 4-carbon with Cl at different positions
- 2 isomers of 3-carbon chain with Cl at different positions
- 1 isomer of cyclobutane with Cl at different positions
- 3 isomers of cyclopropane with Cl at different positions
This sum ups all the structural isomers of C4H7Cl, which are 12 in number as follows:
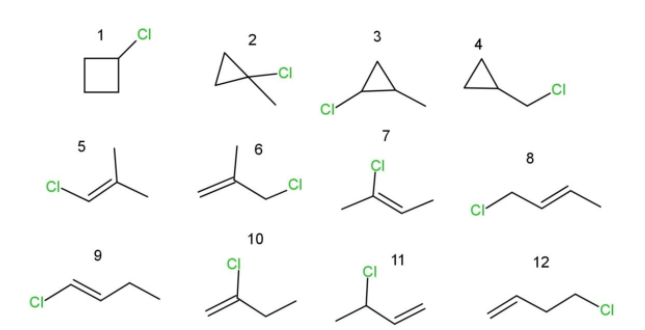
Among these 12 structural isomers, we have some of them as enantiomers and some as diastereomers.
- isomer 3 can have four pairs of enantiomers possible.
- isomer 11 can have two enantiomers possible.
- isomer 2 can have two diastereomers.
- isomers 8 and 9 have two pairs of diastereomers .
- also isomer 3 can have two diastereomers.
This makes the total isomers, including enantiomers and diastereomers, to be 18.
Hence, C4H7Cl have 12 structural isomers, and 6 pairs of enantiomers while 6 pairs of diastereomers , which makes a total 18 isomers.
Note:
Enantiomers and diastereomers, both have a chiral centre and are non-superimposable, but they differ in being mirror images. As enantiomers are mirror images, while diastereomers are not.
