Question
Question: How can I draw the Lewis structure for \(C{{O}_{2}}\)?...
How can I draw the Lewis structure for CO2?
Solution
Hint Lewis structure is the structural representation of the molecule while completing their octet. If you know the rules for drawing the Lewis structure, then, you can easily draw the Lewis structure of any molecule given.
Complete step by step answer:
First of let’s discuss what is Lewis structure. Lewis structure is the representation of the molecule or any compound to depict the structure of that very molecule or compound.
There are certain rules that should be followed to draw Lewis structure of any compound or molecule.
1. First of all, identify the main central atom of the structure. In carbon dioxide, there are two oxygen atoms and one carbon atom, so, it means that carbon atom is the central atom and oxygen atom is attached to it.
2. Now, we will draw a simple basic structure in which the atoms are singly bonded to the main central element as like in carbon dioxide molecule;
O−C−O
3. In, the next step, we will try to complete the valency i.e., the octet of each atom in the molecule by adding double or triple bond in the molecule as;
O=C=O
4. Now, after drawing all the possible structures, we will now complete their octet by adding the valence electrons as;
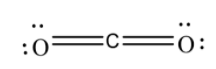
5.After that, we will then check the formal charge of each atom in the structure and if in the structure, all the atoms have the least charge on them, then that structure is the most stable one and is considered as the Lewis structure.
Formal charge in structure is;
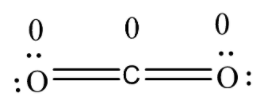
we can see that the structure has the least possible charge on it and thus, is the most stable structure and is regarded as the Lewis structure.
Note: Formal charge is the charge which each atom carries in the molecule . It can be calculated by using the formula as;
F.C=V−N−2B
Here, V represents the number of the valence electrons.
N represents the non-bonding valence electrons
B represents the bonding valence electrons.
