Question
Question: How can I draw the following ethers: 1-propoxypentane, 2-ethoxybutane, 1-methoxy-4-chlorohexane and ...
How can I draw the following ethers: 1-propoxypentane, 2-ethoxybutane, 1-methoxy-4-chlorohexane and 3-butoxy-2,4-dimethyloctane?
Solution
First of all, draw the main chain of the given compound. Then attach the alkoxy group to the particular carbon number mentioned in the name.
Complete step by step solution: Ethers can be categorized as organic compounds where 1 oxygen atom is connected by 2 alkyl or aryl groups. They have the general formula as follows:-
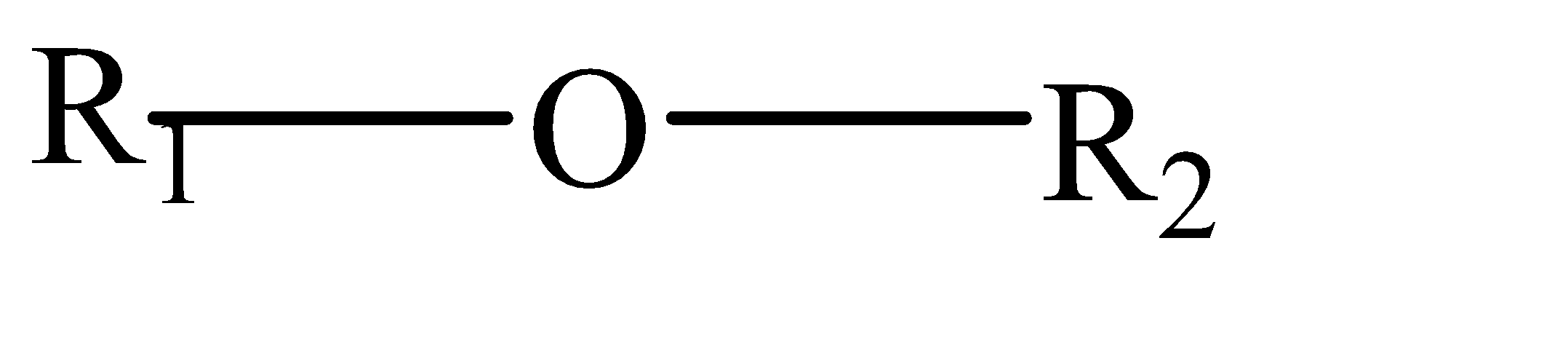
Where R1 and R2 can be aryl as well as alkyl group (same or different).
1-propoxypentane:-
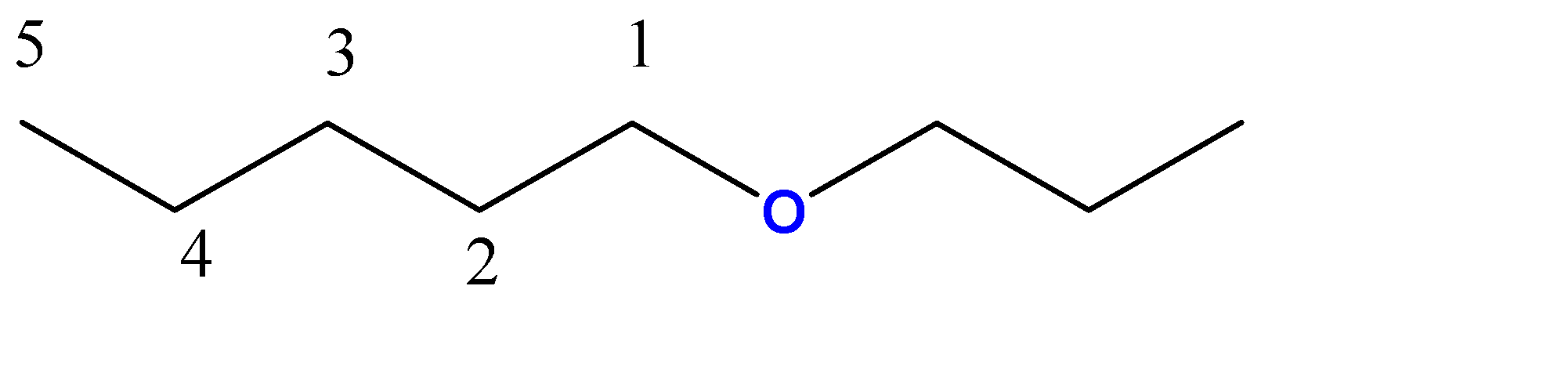
5 carbon atoms chain = pentane. Here, the propoxy group is a 3-carbon chain with an O atom at C1 position.
2-ethoxybutane:-
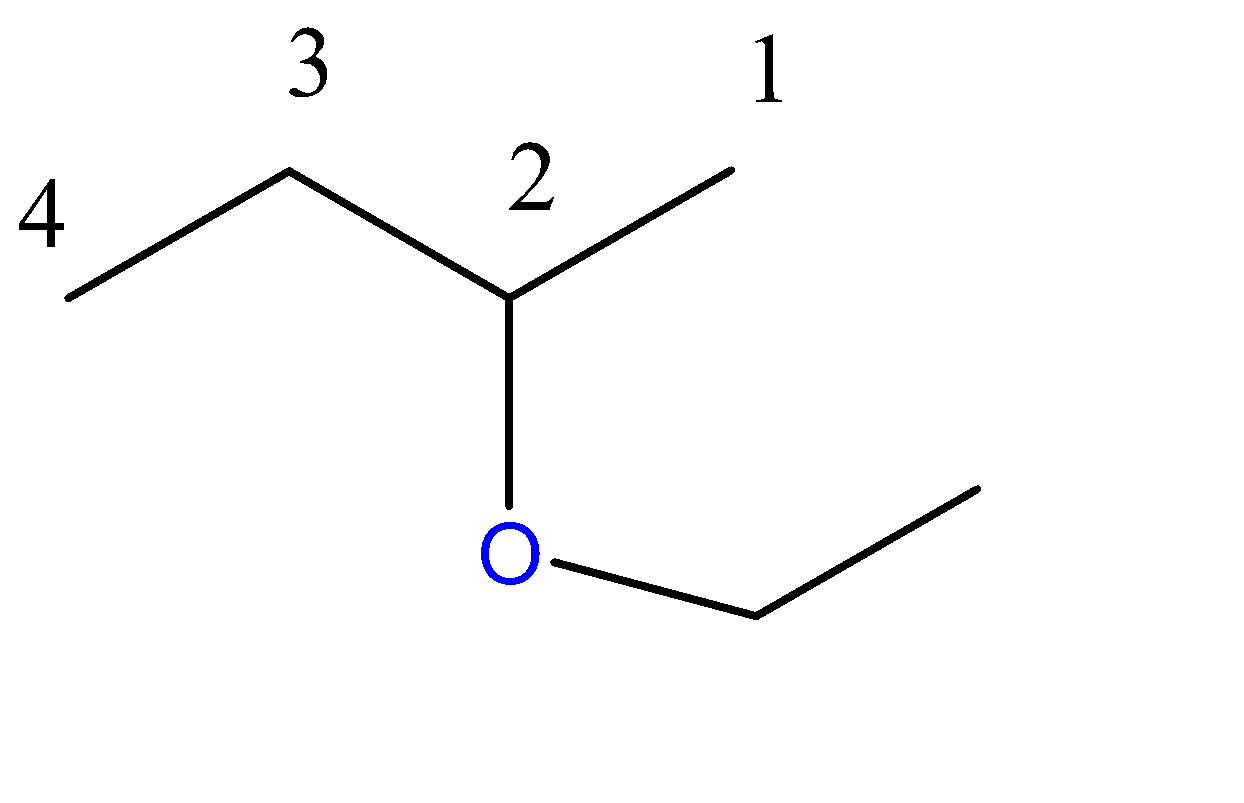
4 carbon atoms chain = butane. Here, the propoxy group is a 3-carbon chain with an O atom at C2 position.
1-methoxy-4-chlorohexane:-
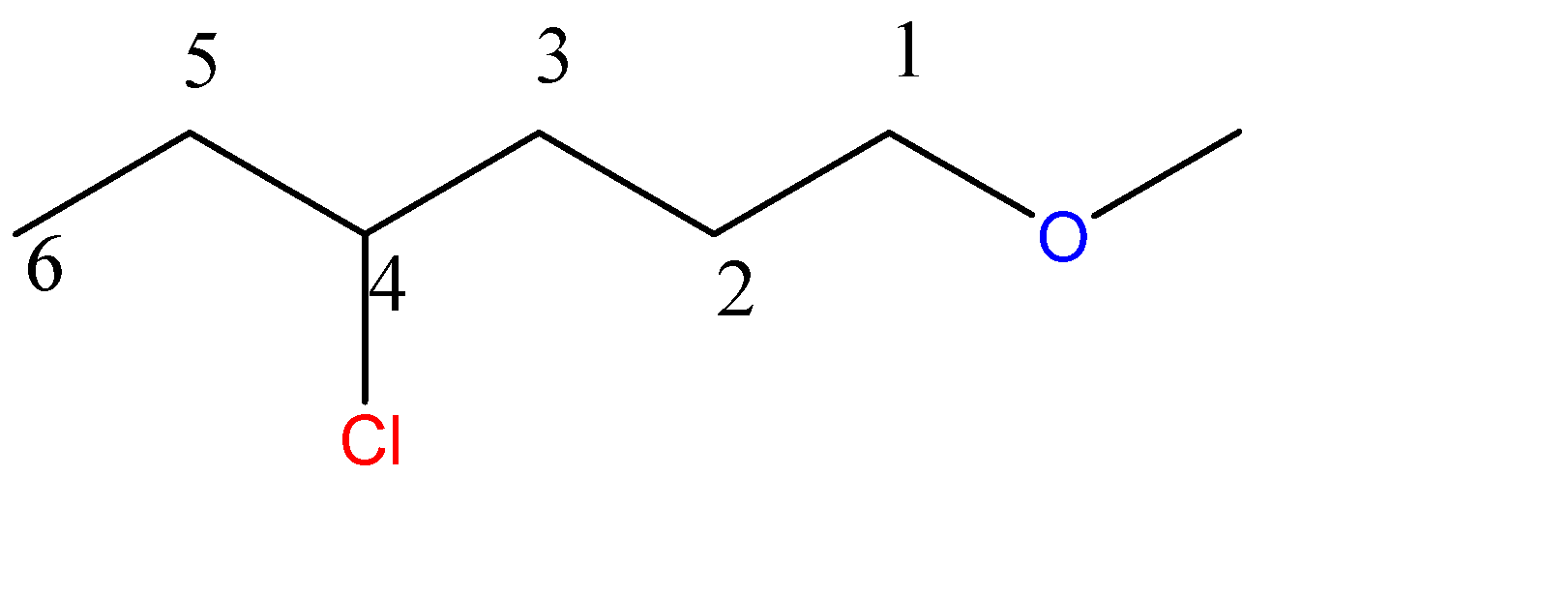
6 carbon atoms chain = hexane. Here, the methoxy group is a 1-carbon chain with an O atom at C1 position and chloro group at C4 position.
3-butoxy-2,4-dimethyloctane:-
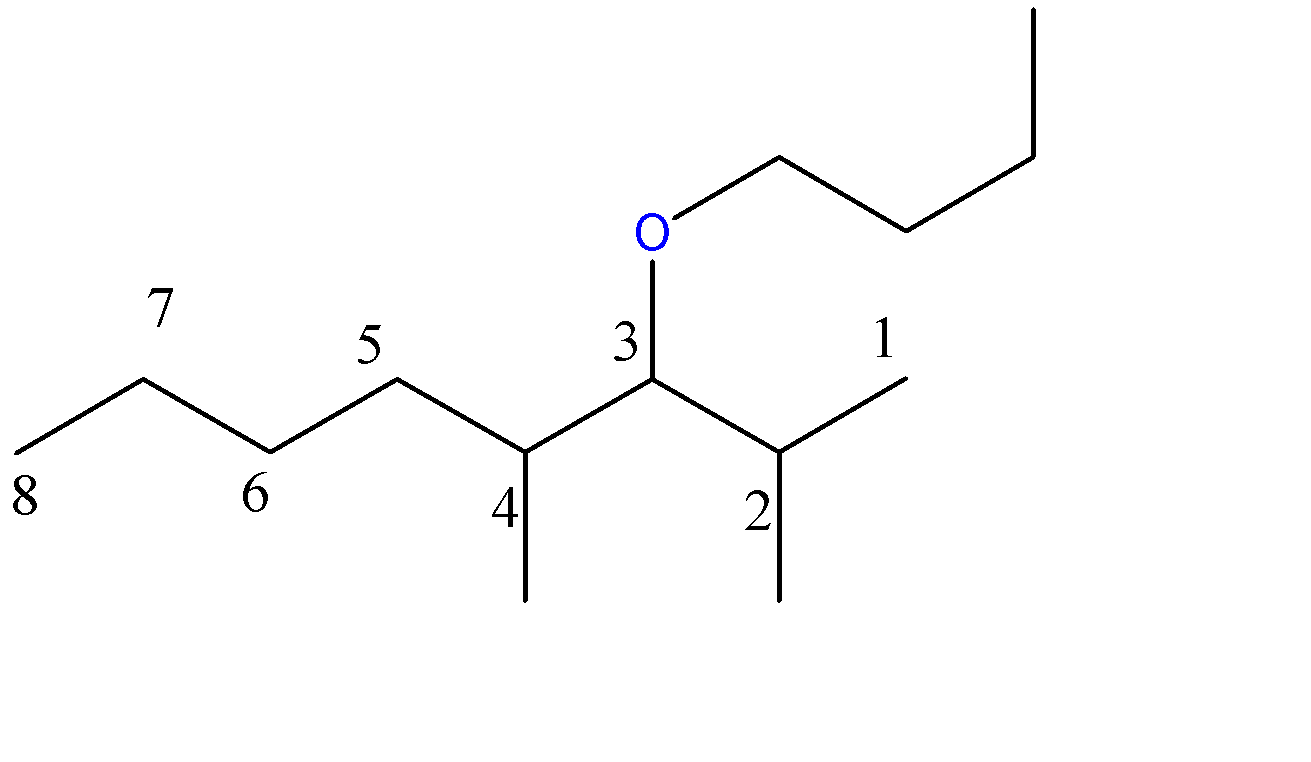
8 carbon atoms chain = octane. Here, the methoxy group is a 1-carbon chain with an O atom at C3 position and 2 methyl groups at C2 and C4 position.
Additional Information:
Ether compounds always contain 1 oxygen atom in it between 2 alkyl/aryl groups. Auto-oxidation of ethers leads to the formation of peroxide compounds. These compounds are used for various purposes especially in fragrant industries. For example: dyes, perfume, scent, fragrant oils and waxes etc.
Note:
Nomenclature of ethers can be done in two ways: a) Common nomenclature prefers to write down the name of all aryl or alkyl groups first(in alphabetical order), followed by the word ‘ether’.
b) According to IUPAC, a substituent with more no. of carbon atoms is chosen as a parent hydrocarbon chain which is used as a suffix whereas the other substituent group attached to O is named as ‘oxy’ which is used as a prefix.
- Always draw the parent hydrocarbon chain at the beginning and give it a number(position) so that later on we can place the right group or substituent on the right carbon position of the parent chain.
