Question
Question: How can I draw pi bonds?...
How can I draw pi bonds?
Solution
If the p – orbital of one atom is going to overlap with the p – orbital of another atom laterally or side wise then the formed bond in between the two atoms is called π - bond or pi -bond. If p – orbital of one atom is going to overlap with the p – orbital of another atom axially then the formed bond in between the two atoms is called π - bond
Complete answer:
- In the question it is asked how to draw pi-bond.
- If two s – orbitals of different atoms are going to overlap axially then there is a chance to form a sigma bond (σ ).
- If one s – orbital of one atom is going to overlap with p – orbital of another atom axially then also there is a chance to form a sigma bond (σ ).
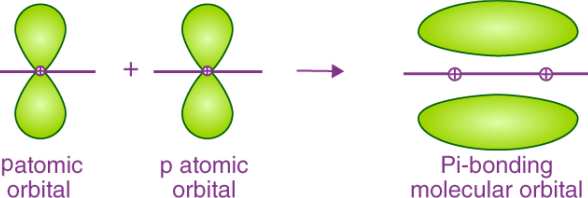
- If two p – orbitals of different atoms are going to overlap sideways then only there is a chance of formation of the pi – bond or π -bond.
- The pi-bond can be represented in the form of an image as follows.
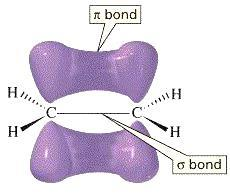
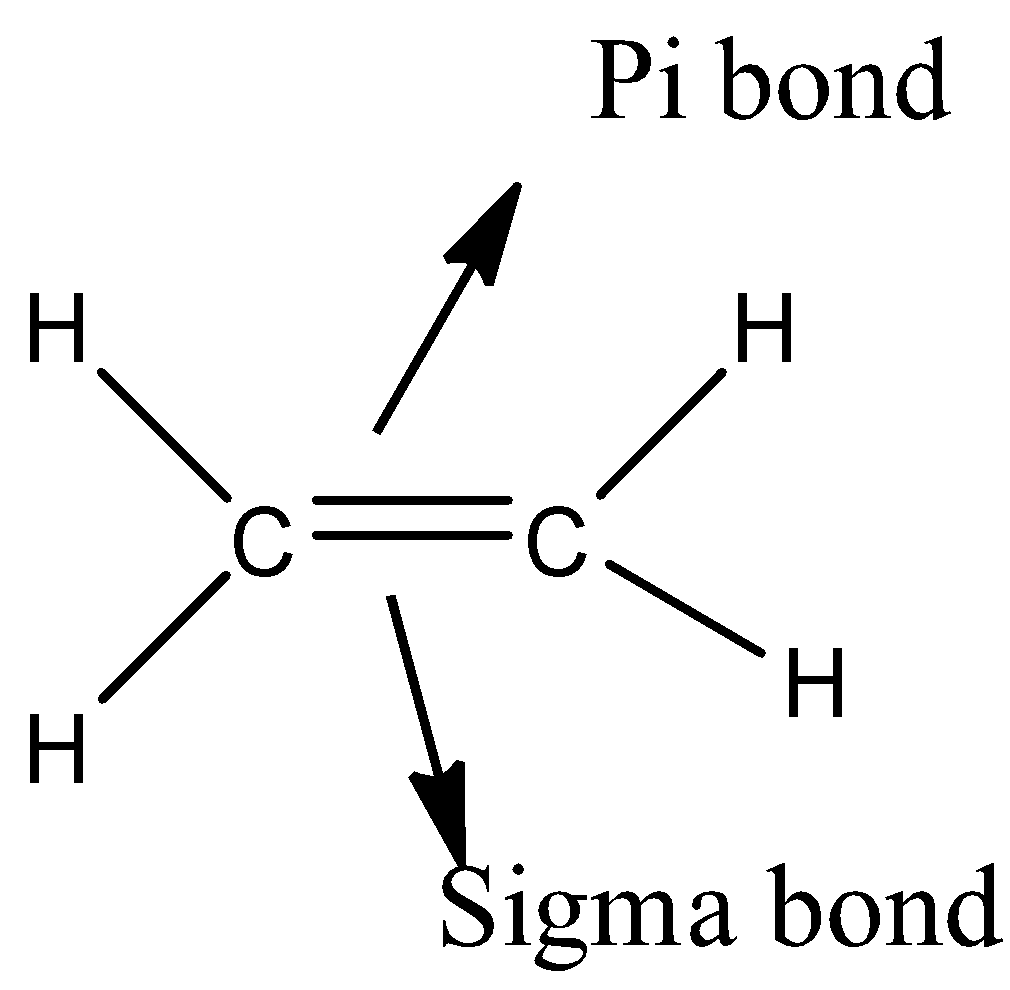
- The molecule which has both a sigma bond and a pi- bond is as follows.
Note:
If a molecule contains one pi-bond means it contains two bonds (One sigma bond and one pi-bond). Double bond is more stable than a single bond because the amount of energy required to break a double bond is more than the amount of energy required to break a single bond.
