Question
Question: How can I draw for cis-1,2-diethylcyclohexane the two chair conformers and indicate which conformer ...
How can I draw for cis-1,2-diethylcyclohexane the two chair conformers and indicate which conformer is more stable?
Solution
First we should know about the axial and equatorial positions in the chair form of cyclohexane to draw the cis-1,2-diethylcyclohexane in chair form. The structure which represents the equatorial and axial positions in cyclohexane is as follows.
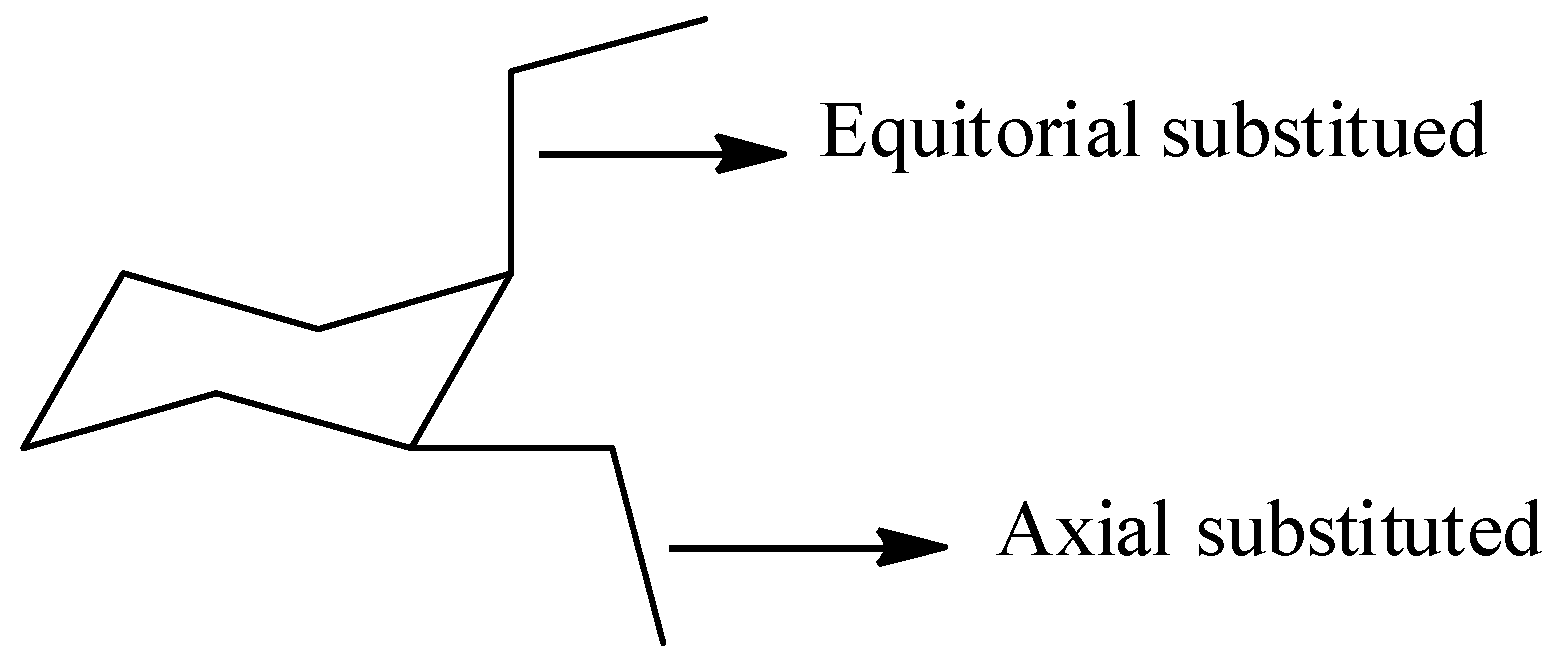
Complete answer:
- In the question it is asked to draw the two chair conformers of cis-1,2-diethylcyclohexane and indicate which conformer is more stable.
- In the question the given molecule is cis-1,2-diethylcyclohexane, means two ethyl groups are cis to each other in chair conformers.
- The possible two chair conformers of the given compound, cis-1,2-diethylcyclohexane is as follows.
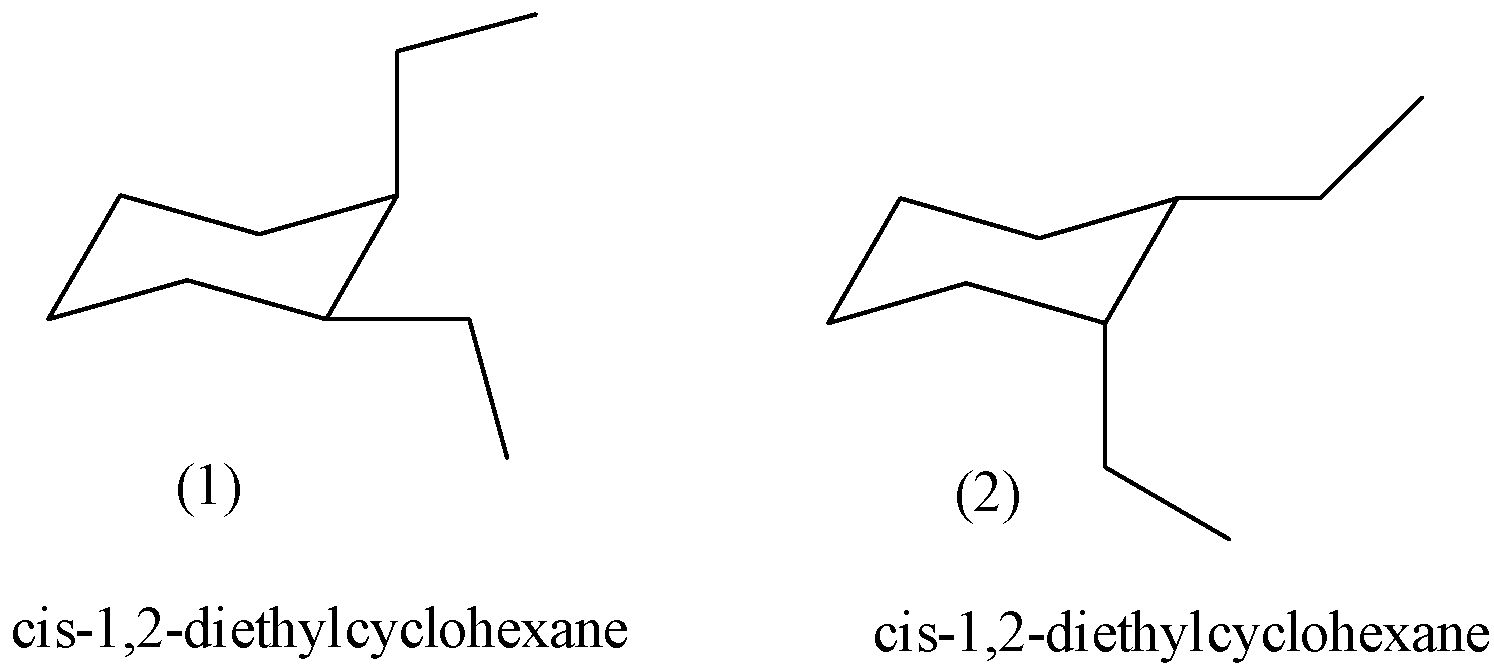
- The above two structures are going to represent the same molecule cis-1,2-diethylcyclohexane.
- In structure- 1, one ethyl group is at equatorial position and other ethyl group at axial position to become cis to each other.
- In structure- 2, one ethyl group is at axial position and other ethyl group at equitorail position to become cis to each other.
- In both the structures the amount of energy going to carry is same by the both the possible chair conformers of cis-1,2-diethylcyclohexane.
- Therefore both the chair conformers are equally stable in nature.
- If one chair conformer is cis and the other is trans then trans chair conformer is more stable than the cis chair conformer.
Note:
In case of chair conformer trans chair conformers are more stable than the cis chair conformers due to the presence of less repulsions in case of trans chair conformer when compared to cis chair conformer.
