Question
Question: how can I draw fischer projections from wedge and dash?...
how can I draw fischer projections from wedge and dash?
Solution
Using wedge and dash notation, solid lines (sticks) represent chemical bonds in the plane of the surface.
Black wedges represent chemical bonds coming toward you, while dashed lines are for bonds that extend back behind the surface
.We must view a wedge-dash formula from the correct angle to convert it to a Fischer projection.
Complete step by step answer:
Here's the wedge-dash structure.
EG GLUCOSE:
We now view the molecule with C−1 at the top and with all chiral carbons closest to our eye.
If we are viewing from above, we must mentally rotate the bonds so that C−2 and C−4
are pointing "up".
When we do this, the wedges become dashes, and the dashes become wedges, as in the picture below.
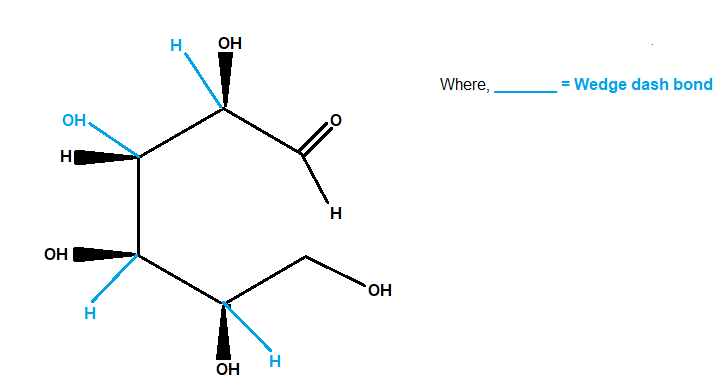
Glucose
So the OH groups on C−2 and C−4 become wedges.
We don't rotate C−3 andC−5 , so the bonds to the OHgroups on those atoms remain the same.
Note: The wedge-dash formula now looks like the one in the image below (I cropped it from here).
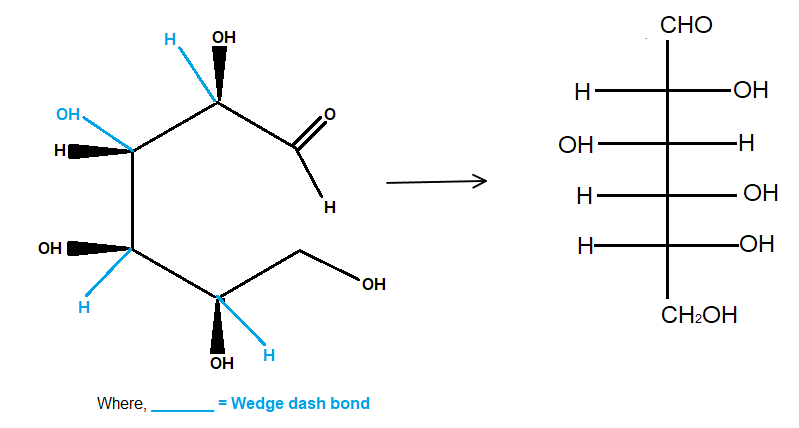 Fischer
Fischer
The wedges are now on the right, and the dashes are on the left.
It is as if we had wrapped the chain around a cylindrical tube.
When you flatten the structure onto the surface of the cylinder, you get the Fischer projection of D-glucose.
understanding the concept of wedge and dash helps to understand stereochemistry and spectroscopy which is an important aspect in research
