Question
Question: How can I draw and identify the chiral centers of 5-deoxyribose?...
How can I draw and identify the chiral centers of 5-deoxyribose?
Solution
The carbon which contains four different groups attached to it in a molecule is called chiral center. The chiral center is optically active and exits in nature in d-form or l-form depending on the rotation of the light.
Complete answer:
- In the question it is asked to draw the chiral centers in 5-deoxyribose molecules.
- To draw the chiral centers in 5-deoxyribose first we should know the structure of D-Ribose.
- The structure of the D-ribose is as follows.
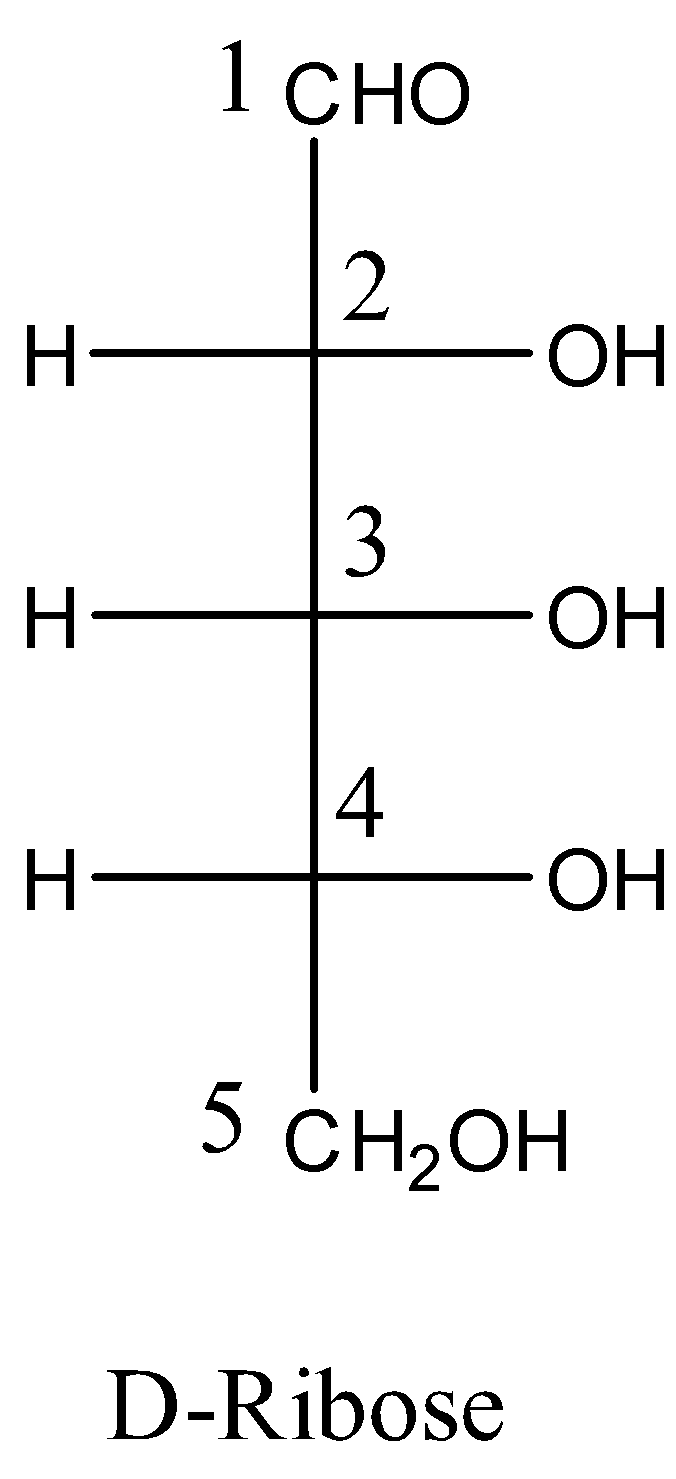
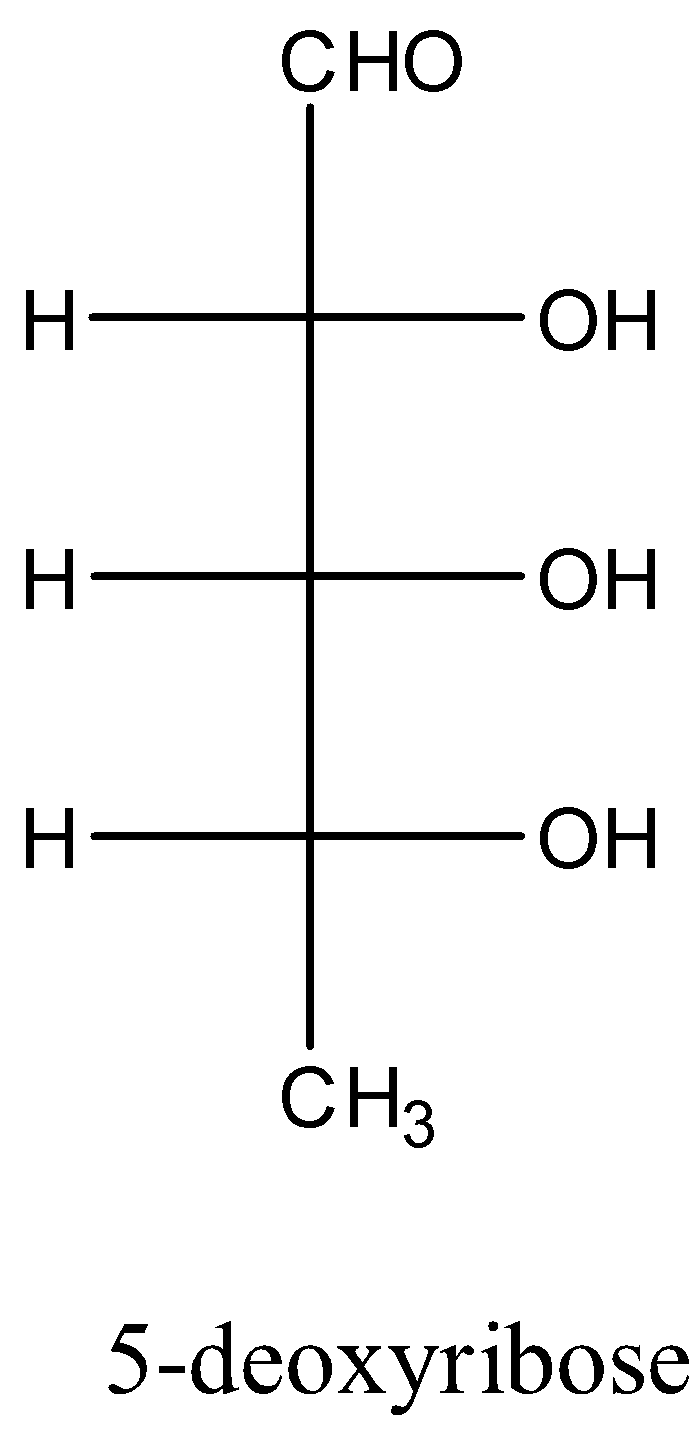
- In the question the given chemical name is 5-deoxyribose means we have to remove one oxygen atom carbon-5 to get the structure of the 5-deoxyribose.
- After removing one oxygen atom at carbon-5 we will get CH3 group in place of −CH2OH .
- The structure of the 5-deoxy ribose is as follows.
- Now we can give the star symbol where chiral carbons are present in the structure of the 5-deoxyribose.
- The structure which contains the chiral centers in 5deoxyribose is as follows.
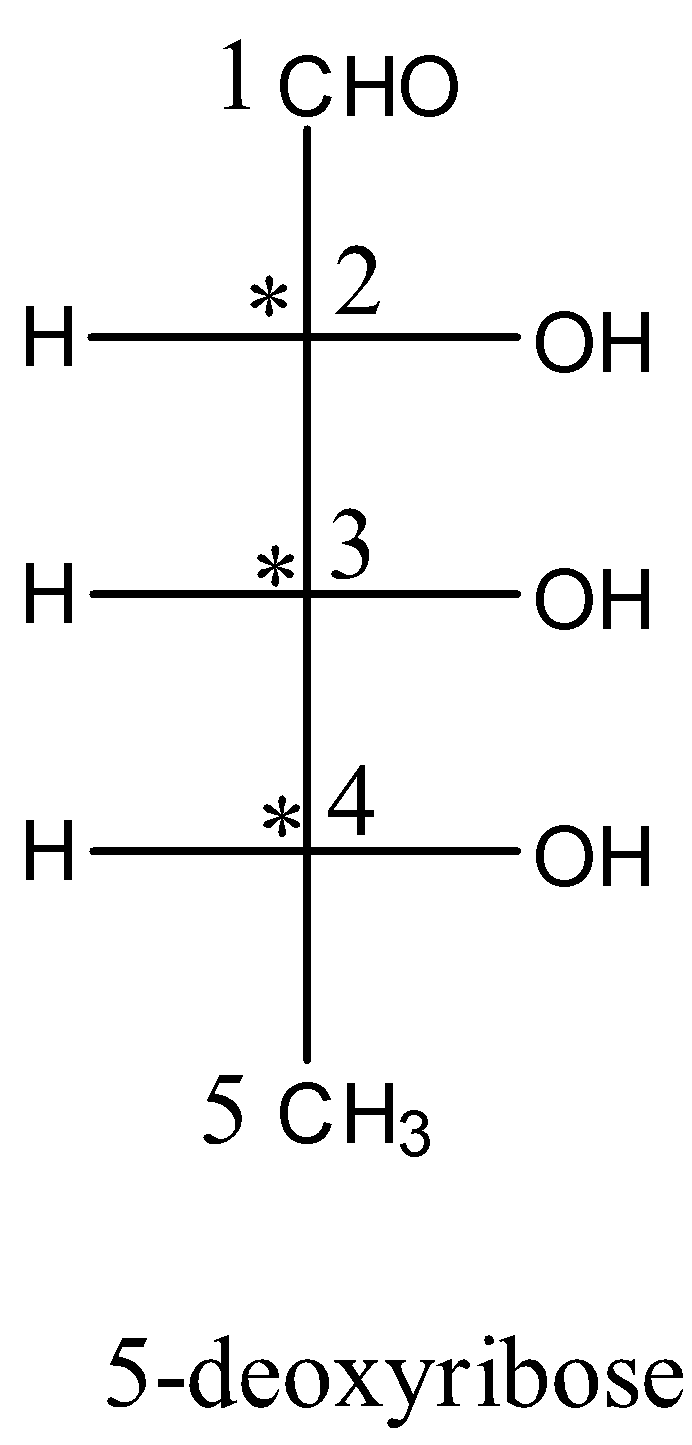
- The carbon-2, carbon-3 and carbon-4 are marked with star marks and they are chiral centers present in the given 5-deoxyribose.
Note:
We have to give numbering in carbohydrates by considering the IUPAC rules. We have to give low numbers to the functional group which are present in the given molecule. If two different functional groups are present in the same molecule we are supposed to give preference to the functional group which contains unsaturation in it.
