Question
Question: How can I draw all possible stereoisomers for 1, 3-Dibromopentane?...
How can I draw all possible stereoisomers for 1, 3-Dibromopentane?
Solution
First, find the number of chiral carbon atoms in the compound, and then we can find the number of stereoisomers that the compound can show. 2n can be used to find the number of stereoisomers where n is the number of chiral carbon atoms in the compound.
Complete answer:
Stereoisomers are those compounds having at least one chiral carbon atom, and there is a different arrangement of atoms in the space having the chemical formula same.
We can define the chiral carbon atom as those carbon atoms in which all the substituents are different. These carbon atoms are represented with a star at the top. Only the carbon atoms having a single bond can be a chiral carbon atom, neither the double or triple bond having a carbon atom be a chiral carbon atom.
Let us see the structure of 1, 3-Dibromopentane-
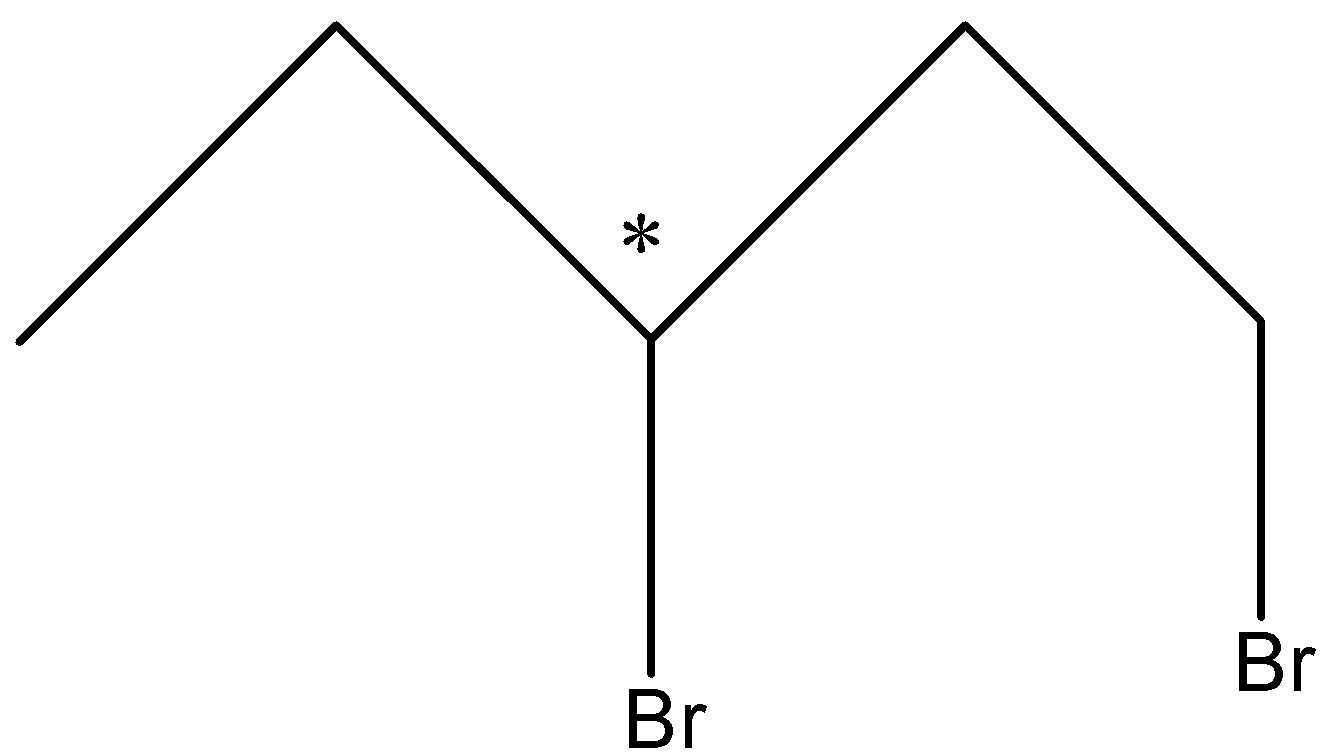
As we can see that in this compound there is only one chiral carbon atom in the compound.
2n can be used to find the number of stereoisomers where n is the number of chiral carbon atoms in the compound. So, putting the value of chiral carbon atoms, we get:
2n=21=2
So, there can be two Stereoisomers of 1, 3-Dibromopentane and these are drawn below-
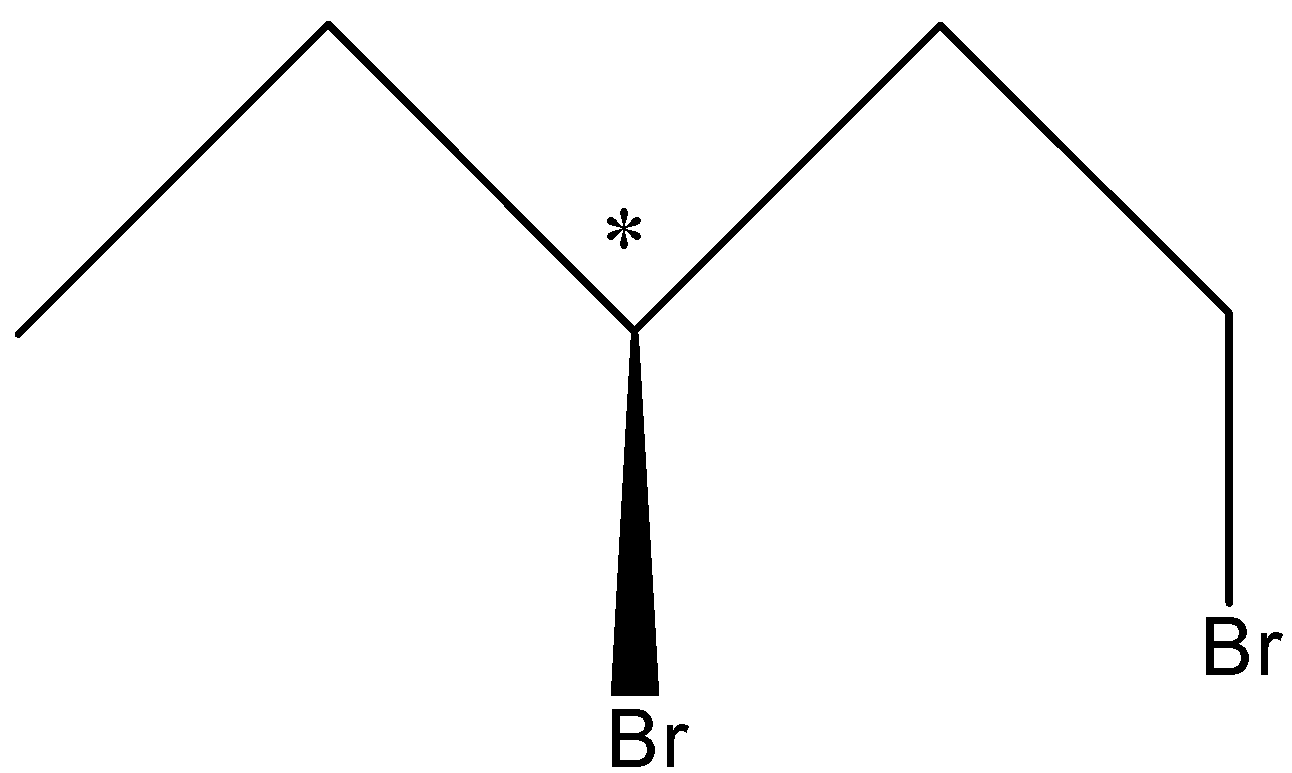
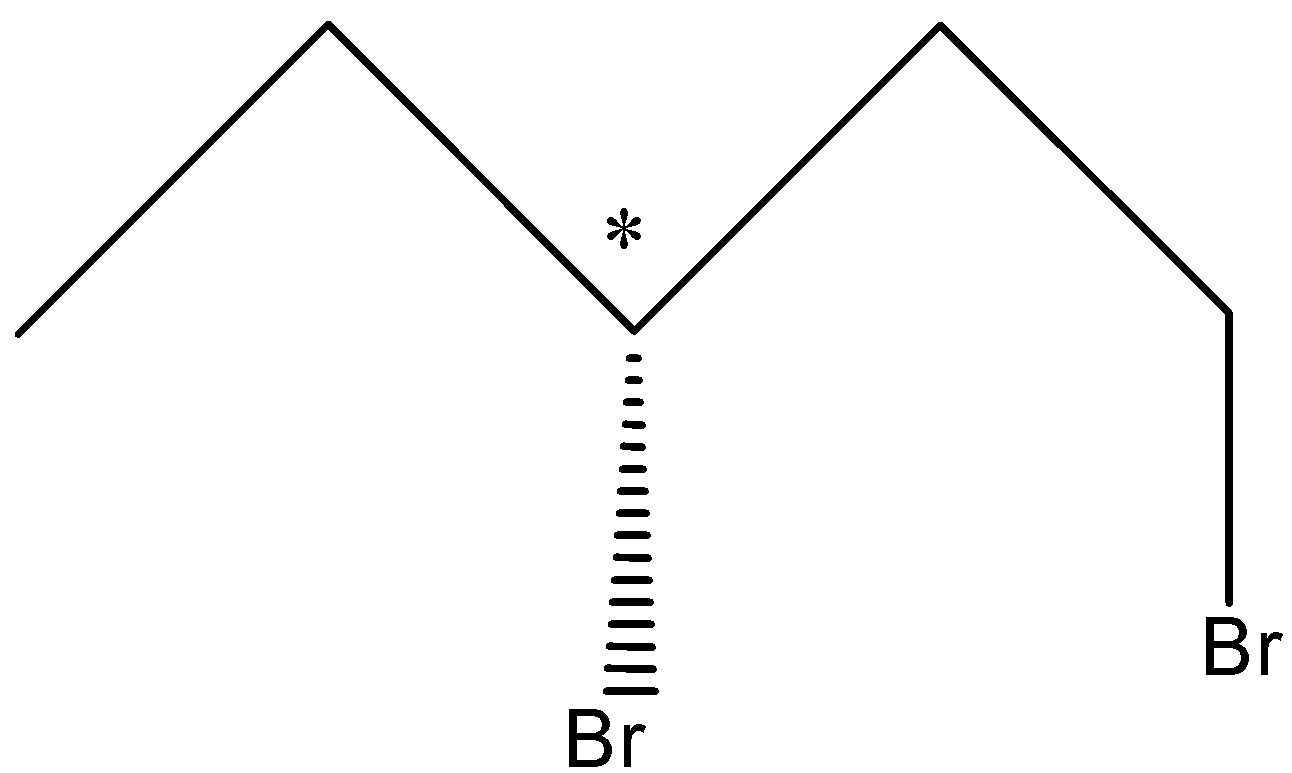
So, in the first structure, the bromine atom is above the plane and in the second structure, the bromine atom is below the plane.
Note:
The atoms that are attached to the chiral carbon atom in the compound will show the stereoisomers. Due to this presence of chiral carbon atom then the compound is said to be an optically active compound.
