Question
Question: How can I convert L-xylose bond line view to Fischer projection?...
How can I convert L-xylose bond line view to Fischer projection?
Solution
We know that using wedge and dash notation, solid lines (sticks) represent chemical bonds in the plane of the surface. Black wedges represent chemical bonds coming toward you, while dashed lines are for bonds that extend back behind the surface.
Complete step by step solution:
A Fischer projection represents every chiral center as a cross. The horizontal line represents bonds extending out of the plane of the paper. The vertical line represents bonds extending behind the plane of the paper. The Fischer projection of L-xylose is
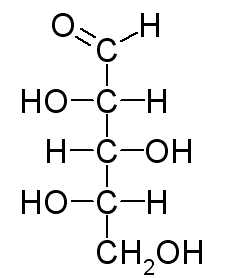
Here's how to convert the wedge-dash structure of L-xylose to its Fischer projection.
Arrange the molecule so that the chiral carbons and the longest continuous chain are in a vertical line. C−1 (the aldehyde group) goes at the top.
Draw horizontal lines to make crosses at C−2, C−3, and C−4.
Put the C−4 OH group on the correct side of the cross. We must view the molecule from the correct angle. This is an L-sugar, so the OH must be on the left, and C−4 must be closest to our eye. We must view the molecule from the lower left. We put the OH on the left arm of the cross. The H atom goes on the right.
Put the C−3 OH group on the correct side of the cross. The OH is on the left, but C−3 is furthest from our eye. We must rotate C−3 to bring it near our eye. The OH then rotates to the right. We put the OH on the right arm of the cross. The H atom goes on the left.
Put the C−2 OH group on the correct side of the cross. The OH group is on the left, and C−2 is closest to our eye. We put the OH on the left arm of the cross. The H atom goes on the right. We now have the Fischer projection of L-xylose.
Note:
Remember that the wedges are now on the right, and the dashes are on the left. It is as if we had wrapped the chain around a cylindrical tube. When you flatten the structure onto the surface of the cylinder, you get the Fischer projection of D-glucose. Understanding the concept of wedge and dash helps to understand stereochemistry and spectroscopy which is an important aspect in research.
