Question
Question: How can I convert a Newman projection for ethane molecule to bond line notation?...
How can I convert a Newman projection for ethane molecule to bond line notation?
Solution
To solve this we must know that the molecular formula for ethane is C2H6. The Newman projection visualizes the conformation of a chemical bond from front to back. In Newman projection, the front atom is represented by a dot and the back atom is represented by a circle.
Complete answer:
We know that the molecular formula for ethane is C2H6.
The Newman projection visualizes the conformation of a chemical bond from front to back. In Newman projection, the front carbon atom is represented by a dot and the back carbon atom is represented by a circle.
Thus, the Newman projection for ethane molecule is as follows:
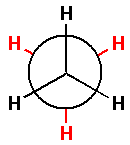
In the Newman projection for ethane molecules, the black hydrogen atoms are attached to the front carbon atom and the red hydrogen atoms are attached to the back carbon atom.
In bond line notation, the bond between two atoms is denoted by a line. In bond line notation, atoms other than carbon and hydrogen are shown.
The steps to convert Newman projection to bond line notation are as follows:
Find the longest chain of carbon atoms.
Locate the substituents.
Now, in the Newman projection for ethane molecules, the longest chain of carbon atoms has two carbon atoms. And each carbon atom has three hydrogen atoms as substituents.
Thus, the bond line notation for ethane molecule is as follows:
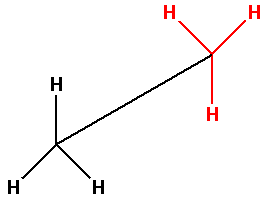
Note: Remember the steps to convert Newman projection to bond line notation are: 1. Find the longest chain of carbon atoms, 2. Locate the substituents. In bond line notation, the bond between two atoms is denoted by a line and only the atoms other than carbon and hydrogen are shown.
