Question
Question: How can I convert \[3 - methylhexane\;\] \[{C_3} - {C_4}\] bond from Newman projection to bond line ...
How can I convert 3−methylhexane C3−C4 bond from Newman projection to bond line notation?
Solution
A Newman plan, supportive in alkane stereochemistry, pictures the variation of an artificial bond from forward-facing to back, with the forward-facing atom rod to by a dot and the vertebral carbon as a circle.
Complete step by step answer:
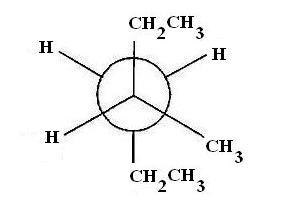
The steadiest conformer for 3−methylhexane saw along the C3−C4 bond has two ethyl bunches in enemy of position and tactless communication between an ethyl gathering and a methyl gathering.
We should decide the gatherings appended to every molecule. In the event that C3is the front atom, you'll see that it has appended the ethyl bunch in the plane of the page, a methyl bunch emerging from the plane of the page, and a hydrogen molecule going into the plane of the page.
The C4carbon is the back carbon and it has joined the ethyl bunch in the plane of the page, and two hydrogen particles out of the plane of the page.
Prior to joining the gatherings to every carbon, you should first draw the parent chain, hexane
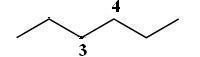
The two ethyl bunches are now appended to the C3 and C4 carbons on the grounds that they’re essential for the parent chain. Since hydrogen atoms are not attracted to bond line documentation, the C4 carbon won't have anything noticeable joined to it.
Nonetheless, the C3carbon will have the methyl gathering − CH3 − joined and obvious in the documentation.
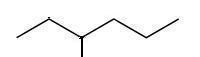
that is the bond line documentation for 3−methylhexane.
Note:
The most steady conformer for 3−methylhexane saw along the C3−C4 bond has two ethyl bunches in enemy of position and tasteless connection between an ethyl gathering and a methyl gathering.
