Question
Question: How can diastereomers be separated?...
How can diastereomers be separated?
Solution
We know that diastereomers are the stereoisomers that are not superimposed on each other and they are not the mirror images of each other. They have two or more stereocenters.
Complete step by step answer:
Let’s understand diastereomers with the help of an example.
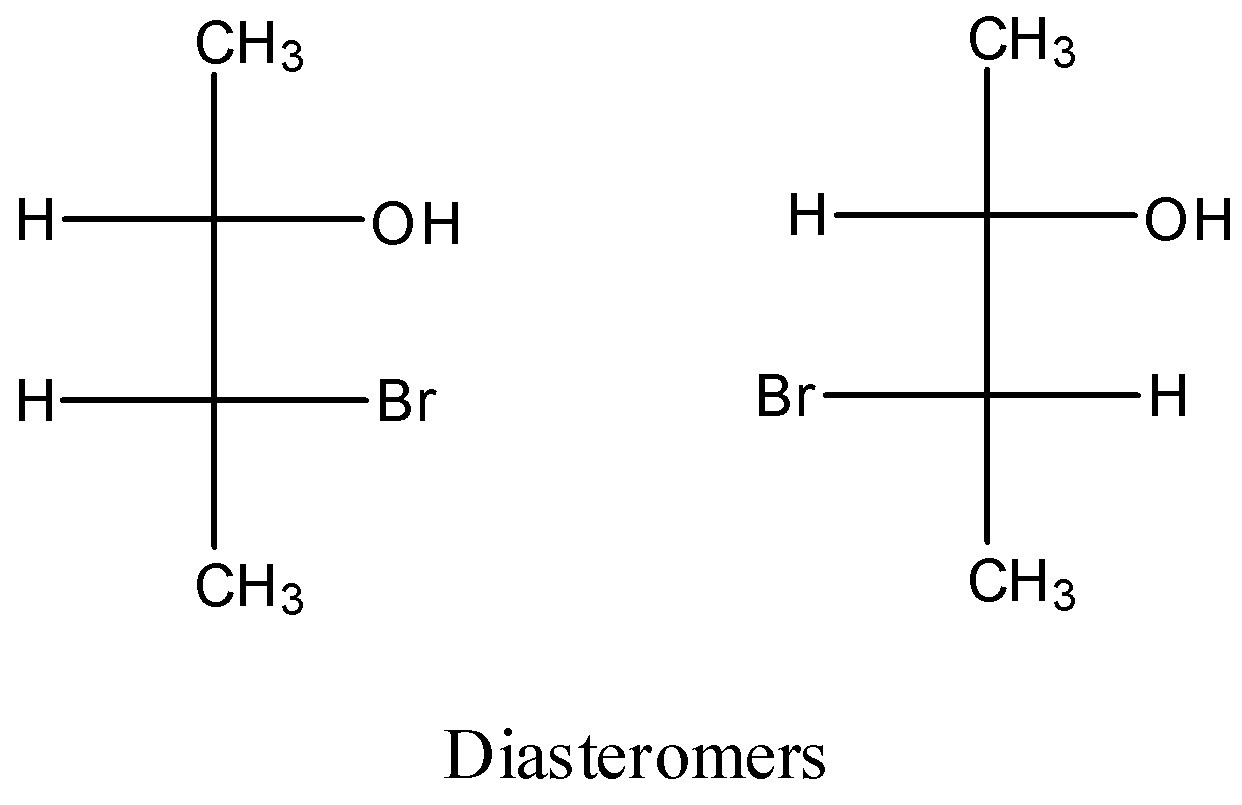
The above two compounds are diastereomers as both the not the mirror images and superimposed on each other.
Let’s discuss the physical properties of diastereomers. They have different physical properties such as melting point, boiling point etc. The reason for identical physical properties of diastereomers is because of their different physical shape.
Now, we will discuss the separation of diastereomers. As diastereomers have different physical properties they can be separated by ordinary methods of separation such as fractional crystallization.
Additional Information:
Stereoisomerism is the phenomenon in which compounds have the same molecular formula as well as the same structural formula but differing in the relative arrangement of the atoms or groups in space are called stereoisomers and the phenomenon is called stereoisomerism.
Let’s discuss enantiomers in detail. Enantiomers are a pair of molecules that exist in two forms which are mirror images of each other. But they are not superimposed on each other. They have identical chemical and physical properties. Due to identical physical properties, they cannot be separated by ordinary methods of separation such as crystallization, distillation etc.
Note: It is to be noted that in the fractional crystallization method, diastereomers are separated on the basis of their solubility. Column chromatography is another method used to separate diastereomers. In this method diastereomers have different degrees of attraction to a stationary phase.
