Question
Question: How can a Peptide Bond be identified?...
How can a Peptide Bond be identified?
Solution
Hint : We need to know that the peptide bond is a bond that links two alpha-amino acids. The linkage occurs between C1 of one alpha- amino acid to N2 of the adjacent amino acid through a protein or peptide chain. It is also called a peptide bond so as to differentiate it from the isopeptide bonds, which is another type of bond between amino acids.
Complete answer:
We need to know that the peptide bond is a bond formed between two amino acids when the carbonyl group of one amino acid interacts with another molecule’s amino group. Water molecules are eliminated resulting in a CO−NH bond which is the peptide bond. The resulting molecule formed is an amide. Peptide bond is a variant of amide bond that connects peptide or protein chain via C1 of one alpha amino acid to the N2 of another alpha amino acid.
The process of formation of peptide bonds is a condensation reaction resulting in removal of water (dehydration). The reaction of shown below:
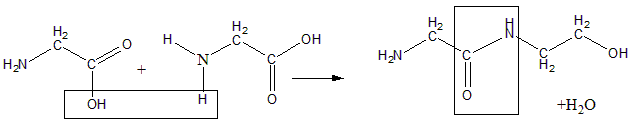
The peptide bond formation consists of three steps:
Two amino groups are brought together such that the acid group of one is close to the amine group of the other.
Water molecule is eliminated leaving a bond between acid carbon and amine nitrogen of second.
The [peptide bond is left between the two amino acids.
The ribosomes are macroscopic and complex cell structures which consist of proteins, RNA and Numerous other components. Peptide bond is one of the strongest and stable bonds that connects amino acids. The formation of peptide bonds requires energy which is given by ATP. Organisms use enzymes to produce nonribosomal peptides, and ribosomes to produce proteins.
Note:
We need to remember that a peptide bond can be broken by hydrolysis. The process is very slow with half life of 350−600 years per bond. In living organisms’ enzymes like Peptidases or proteases catalyze this process.
