Question
Question: How are the graphs for A. Boyle’s Law B. Pressure Law C. Charles law...
How are the graphs for
A. Boyle’s Law
B. Pressure Law
C. Charles law
Solution
Boyle’s Law gives the relation between pressure and volume when the temperature is constant. Therefore, Boyle’s Law gives the graph for pressure versus volume. Charles Law gives the relation between volume and temperature of a gas at constant pressure. It gives a graph of temperature versus volume. Similarly, Pressure Law gives the relation between pressure and temperature at constant volume. Therefore, it gives the graph of pressure versus temperature.
Formula Used:
Boyle’s Law is given as: V∝P1 at constant temperature
Charles Law is given as: V∝T at constant pressure
Pressure Law is given as: P∝T at constant volume
where, Vis the volume, P is the pressure and T is the temperature.
Complete step by step answer:
Boyle’s Law, Pressure Law and Charles law are the three gas laws. The properties of a gas depends upon pressure P, temperature T and volume Vof the gas.
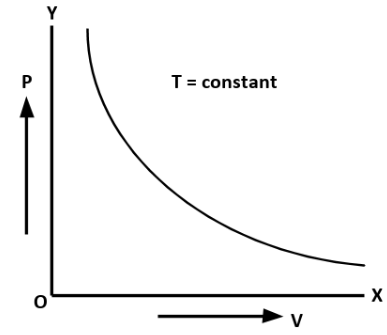
A. Boyle’s Law: At constant temperature for a given mass of ideal gas, the volume of the gas is inversely proportional to its pressure.
V∝P1
where, Vis the volume and P is the pressure.
The graph between pressure and volume at constant temperature is known as isotherm.
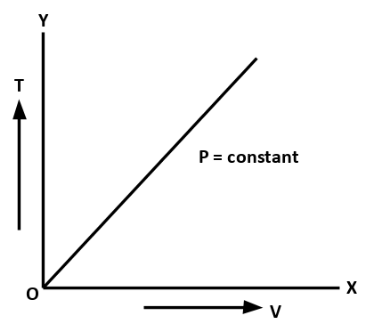
B. Charles Law: At constant pressure, volume of a given mass of gas is directly proportional to its absolute temperature.
V∝T
The curves between volume and temperature at constant pressure are called Isobars.
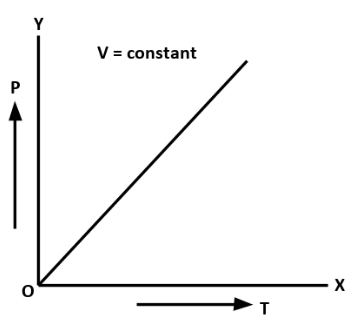
C. Pressure Law: At constant volume, the pressure of a given mass of an ideal gas is directly proportional to the absolute temperature of the gas.
P∝T
The graph for pressure law is as follows
Note: In Boyle’s law, the volume of a given gas is inversely proportional to its pressure. Therefore, pressure times volume will be a constant term. That is PV=constant. The pressure law is also known as Gay Lussac’s Law. Note that the Boyle’s Law, Pressure Law and Charles law are only applicable to Ideal gases. Ideal gas is the perfect gas. But, the behaviour of real gases at high temperature and low pressure is very similar to that of the ideal gas.
