Question
Question: How are the following conversions carried out? Propene \[\to \]Propan-2-ol...
How are the following conversions carried out?
Propene →Propan-2-ol
Solution
In the given reaction an alkene is going to convert into alcohol without changing its number of carbons in the reactant. The reaction is only possible by using sulphuric acid as a reagent.
Complete step by step solution:
- The conversion of propene to propan-2-ol is a two-step process.
The complete reaction is as follows.
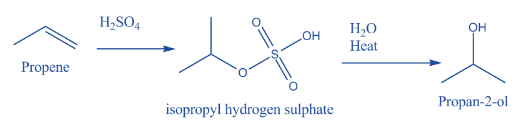
- The above reaction is a two-step reaction.
- In the first step sulphuric acid reacts with propene and forms an intermediate compound. The name of the intermediate compound is isopropyl hydrogen sulphate.
- In the second step the formed isopropyl hydrogen sulphate converted into propan-2-ol by the presence of water and heat.
It is a type of additional reaction because alkene is converted into saturated compounds (alcohol).
The formation of the product is easily confirmed by the sweet smell.
Alcohols with small chains given sweet smell during their synthesis.
So, the conversion of propene to propan-2-ol is possible by the hydration of propene in presence of sulphuric acid.
Propan-2-ol is also called Isopropyl alcohol.
Isopropyl alcohol is a colorless, flammable chemical compound with a strong odor.
In the isopropyl alcohol hydroxyl group linked to second carbon, it is the simplest example of a secondary alcohol.
Note: Don’t be confused with the words alkene and alcohol.
Alkene contains double bonds in its structure.
Alcohol contains an −OH functional group in its structure.
