Question
Question: How are the following conversions brought? i.benzyl chloride to benzyl alcohol ii. benzyl alcoho...
How are the following conversions brought?
i.benzyl chloride to benzyl alcohol
ii. benzyl alcohol to benzoic acid
Solution
The concept of nucleophilic substitution reaction as well as the oxidation of organic compounds to form aldehydes/ketones or carboxylic acids is to be used in this question.
Complete Solution :
- In order to answer this question, we need to learn about the nucleophilic reaction as well as the oxidation reaction, to form acids. Nucleophilic substitution bimolecular or SN2 is a single step bimolecular reaction in which the incoming nucleophile attacks the C-atom of substrate in a direction opposite to the outgoing nucleophile. The reaction passes through a transition state in which both the incoming and outgoing nucleophiles are bonded to the same C-atom. In the transition state, the C-atom is sp2 hybridised with a p-orbital whose one lobe overlaps with an orbital of incoming nucleophile and the other lobe overlaps with an orbital of outgoing nucleophile. The three non-reacting atoms or groups attached to the C-atom are nearly coplanar at an angle of 1200. The reaction is completed when the outgoing nucleophile leaves with the bond pair of electrons and simultaneously the incoming nucleophile binds to the C-atom. As the reaction progresses, the configuration of the C-atom under attack is inverted. An SN2 reaction is always accompanied by inversion of configuration. The inversion in configuration implies change in configuration from R to S or S to R (provided the incoming nucleophile and outgoing nucleophile have same priority) and not from (+) to (-) or (-) to (+).
- Primary alcohols are easily oxidised to carboxylic acids with oxidants such as KMnO4, in neutral, acidic or alkaline media or by CrO3/K2Cr2O7 in acidic media. Now, let us come to the question and see the reactions. In (i), an SN2 reaction is taking place.
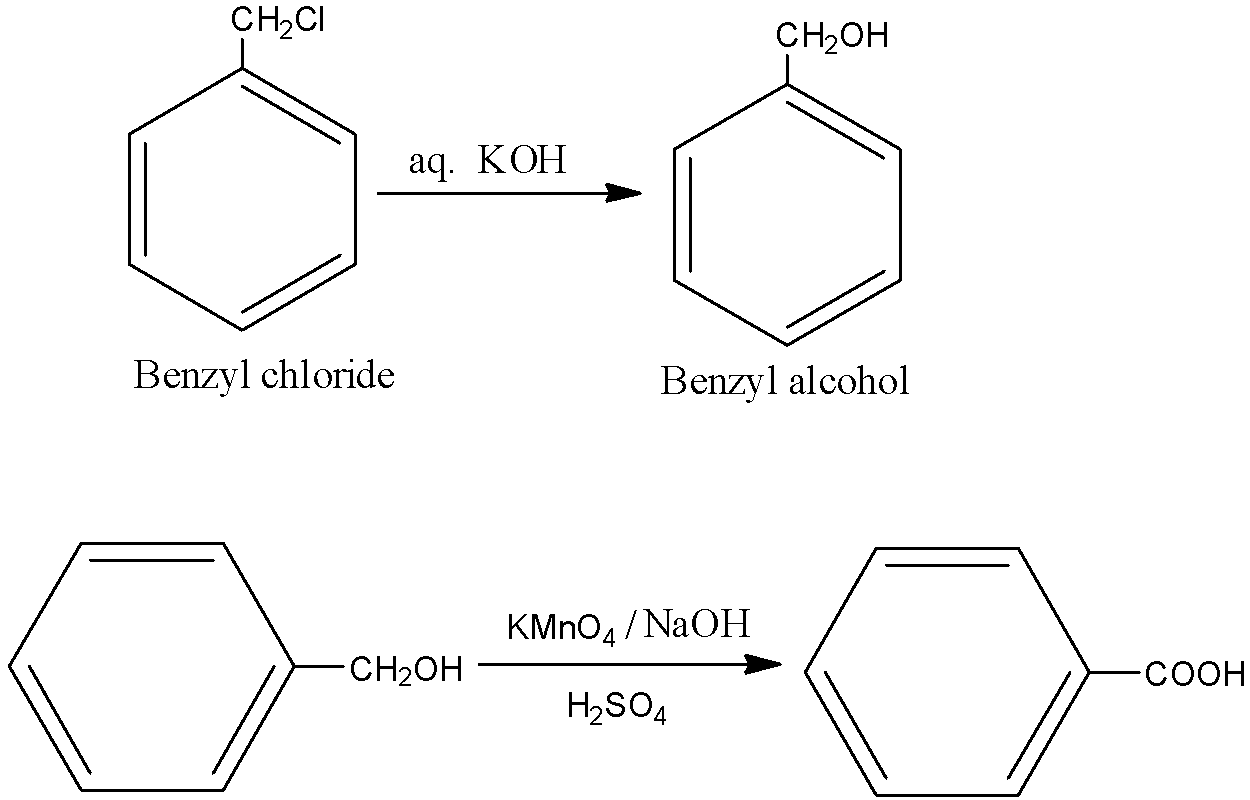
Hence with the above reagents, we obtain the desired products.
Note: Steric hindrance plays a very vital role in an SN2 reaction. As steric hindrance increases the rate of SN2 reaction decreases. Thus for the same halogen the reactivity order of alkyl halides towards SN2 reaction is as under.
- Methyl halide > primary Alkyl halide > secondary Alkyl halide > tertiary Alkyl halide
