Question
Question: How are the following compounds prepared? Benzaldehyde from benzoyl chloride...
How are the following compounds prepared? Benzaldehyde from benzoyl chloride
Solution
The acid chlorides can be converted into the aldehyde through catalytic hydrogenation .Catalytic hydrogenation is a process of addition of hydrogen across the compound. The general reaction for the conversion of acid chloride to the aldehyde in presence of a metal catalyst is as shown below,
R−COClH2Pd/BaSO4 R−CHO
Complete step by step answer:
An aldehyde is a functional group(−CH=O). It can be introduced on organic molecules through various methods. One of the methods involves the preparation of aldehyde from the acid chlorides.
The reaction in which acid chlorides (R−COCl) are converted by the catalytic hydrogenation in the presence palladium (Pd) catalysts supported on the barium sulphate is called as the Rosenmund reduction reaction.
It favors the preparation of aldehyde only.
The general reaction is as depicted below:
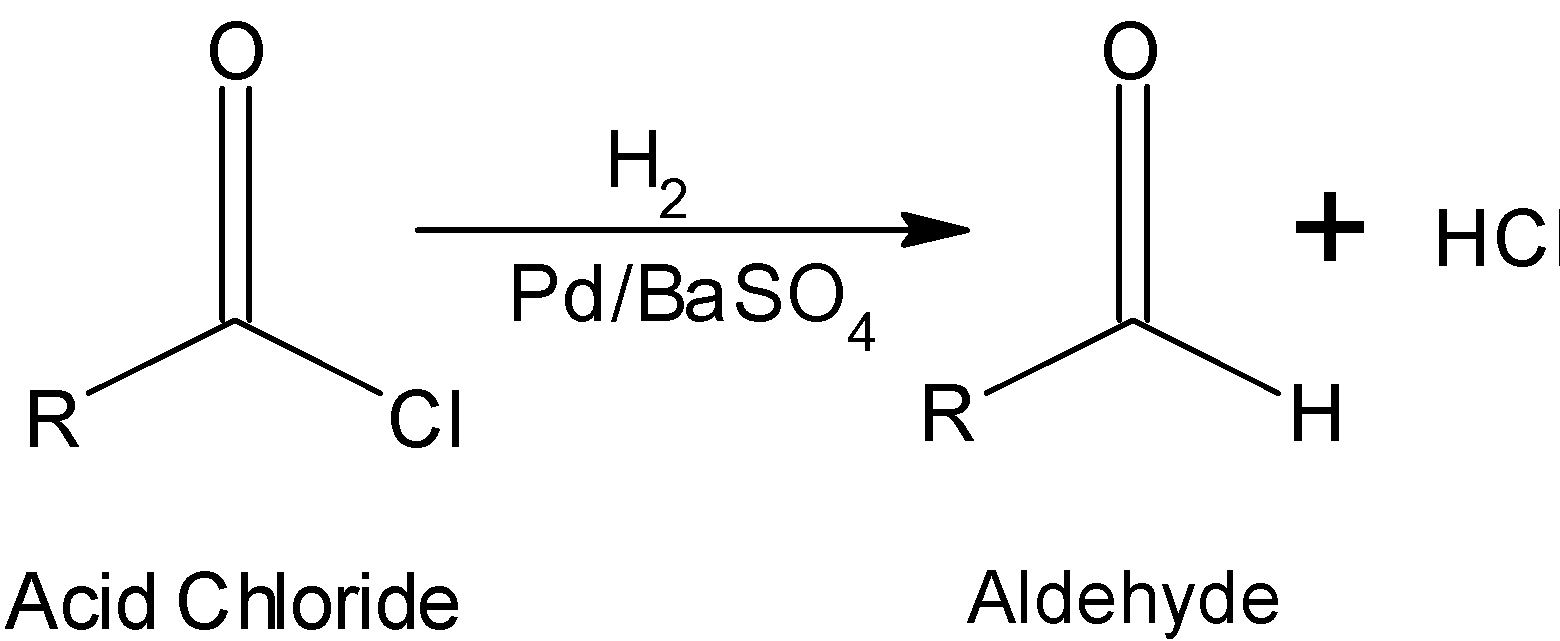
The mechanistic approach for the conversion of acid chloride to the aldehyde is as shown below:
Step 1) Hydrogen gas in presence of the Rosenmund catalyst Pd/BaSO4 is passed through the acyl or acid chloride. This results in the formation of the insertion of palladium next to the carbonyl group.
Step 2) the hydrogen gas breaks down and palladium is removed followed by the hydrogenation of the acyl chloride to the aldehyde.
Step 3) further, the same reaction can take place for the conversion of the carbonyl group to the alcohol (Extended reduction). However, we are interested in aldehydes only. Thus, the catalyst is poisoned by the addition of a small amount of sulphur or quinolone.
Mechanism:
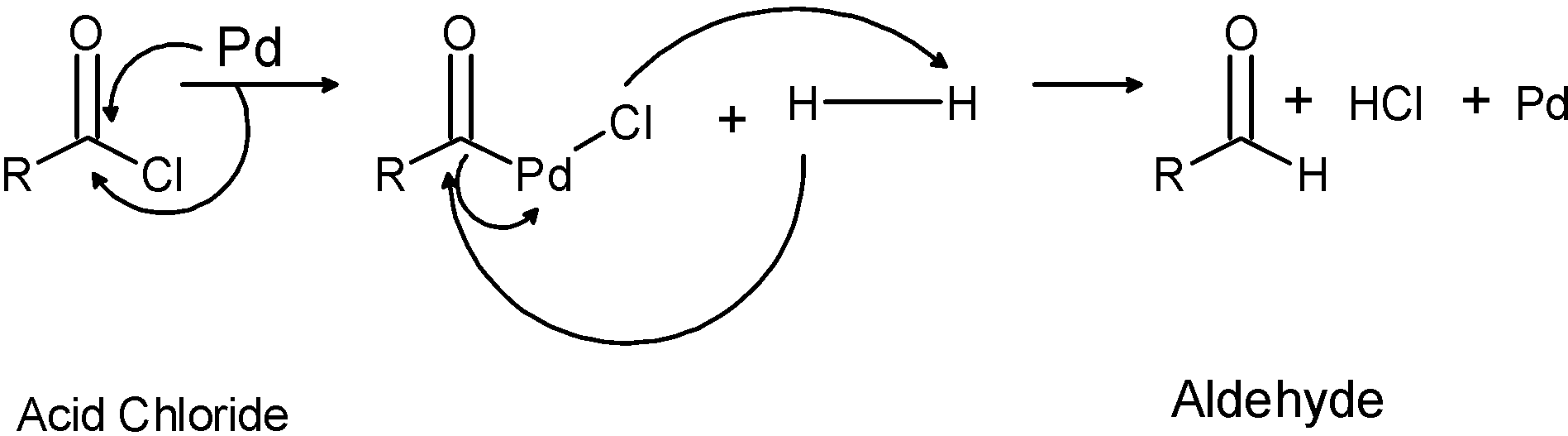
Normally, aldehydes are further reduced to a primary alcohol. Therefore, to prevent further reduction of aldehydes, the catalytic mixture is poisoned (deactivated) by the addition of sulphur or quinoline. This controls the reaction to aldehyde only.
Let’s consider an example of benzoyl chloride. When benzoyl chloride is treated with the hydrogen and palladium on barium sulphate, the chloride group is hydrogenated to give out the benzaldehyde as the product. The reaction is as follows:
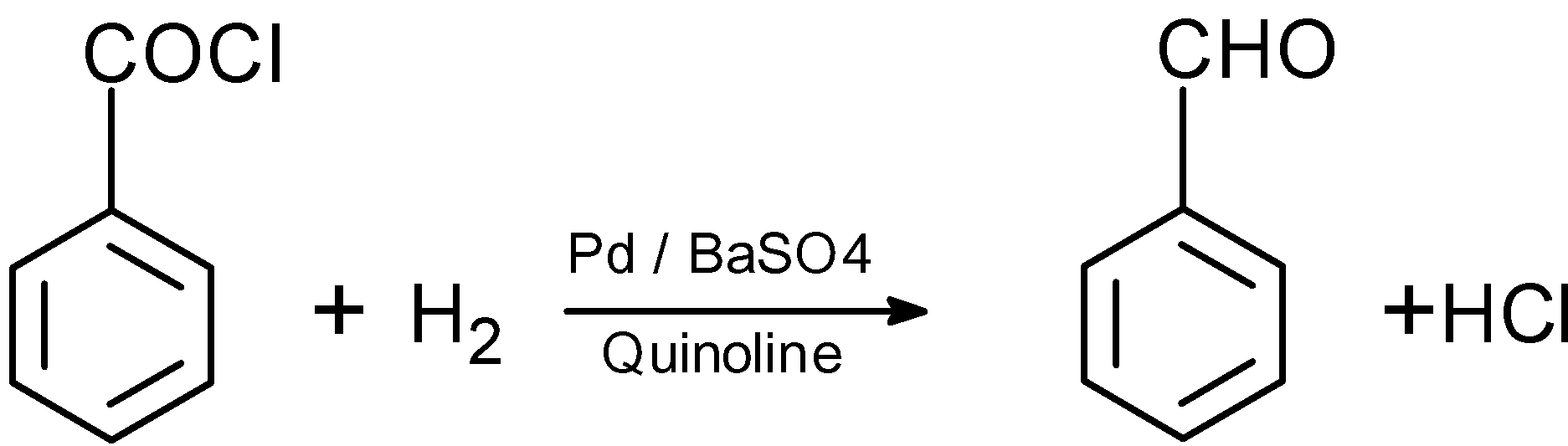
Thus, here we have successfully converted the benzoyl chloride into the benzaldehyde.
Note: Note that formaldehyde cannot be prepared by the Rosenmund reduction reaction. This is because its corresponding acid chloride that is formerly chloride ( HCOCl ) is unstable at room temperature thus does not undergo the reduction reaction. Alternatively, the acid chlorides can be converted to the aldehyde by lithium tri-tert-butoxyaluminum hydride [LiAlH(O−t−Bu)3] at −780C .
