Question
Question: How are \({\text{Xe}}{{\text{O}}_3}\) and \({\text{XeO}}{{\text{F}}_4}\) prepared? Give the structur...
How are XeO3 and XeOF4 prepared? Give the structures.
Solution
Xenon is a gas that is used in flash and arc lamps, as well as as a general anaesthetic. The first excimer laser used a xenon dimer molecule as the lasing medium, and the first laser pumps were xenon flash lamps. Xenon is used as a propellant for ion thrusters in satellites and to scan for possible weakly interacting large particles.
Complete answer: The chemical element xenon has the symbol Xe and the atomic number 54. It is a colourless, thick, and odourless noble gas present in trace concentrations in the Earth's atmosphere. Xenon can undergo a few chemical reactions, including the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesised.
In its +6 oxidation state, xenon trioxide is an acidic xenon compound. It's a powerful oxidizer that steadily releases oxygen from water, which is accelerated by exposure to sunlight. When it comes into contact with organic products, it becomes dangerously explosive. It emits xenon and oxygen gas as it detonates.
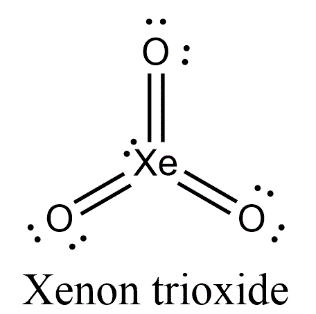
Fluorides of xenon always react with water vigorously to form XeO3
Xenon hexafluoride XeF6+ Water 3H2O→ Xenon trioxide XeO3+6HF
Xenon tetrafluoride 3XeF4+ Water 6H2O→ Xenon 2Xe+ Xenon trioxide XeO3+12HF+121O2
Sometimes Partial hydrolysis of XeF6 yields XeOF4
The chemical compound xenon oxytetrafluoride is an inorganic compound. It's a colourless, solid liquid with a melting point of −46.2oC that's made by partially hydrolyzing XeF6.
Xenon hexafluoride XeF6+ Water H2O→ Xenon oxytetrafluoride XeOF4+ Hydrogen fluoride 2HF

Note:
Seven stable isotopes and two long-lived radioactive isotopes make up naturally occurring xenon. More than 40 unstable xenon isotopes decay radioactively, and xenon isotope ratios are a useful instrument for researching the Solar System's early history. The most important (and unwanted) neutron absorber in nuclear reactors is radioactive xenon-135, which is formed by beta decay from iodine-135 (a result of nuclear fission).
