Question
Question: How are structural isomers drawn?...
How are structural isomers drawn?
Solution
Isomers are molecules that have the same molecular formula but have a different structural arrangement of the atoms in space. This is carried out by rearranging the positions of the different atoms present in the compound.
Complete answer:
- Compounds which show isomerism due to difference in their structures are known as structural isomers. This phenomenon is known as structural isomerism.
- The different arrangements of structural isomers are possible due to the molecule rotating as a whole or whole or rotating about particular bonds.
- In structural isomerism, the molecular formula is the same and the order of arrangement of atoms are different. This can be understood easily with the help of some examples.
- Alkenes are the simplest class of organic compounds that show structural isomerism. They contain only tetravalent carbon atoms who can arrange themselves in different orientations. Butane, methylpropane etc. are some examples for this.
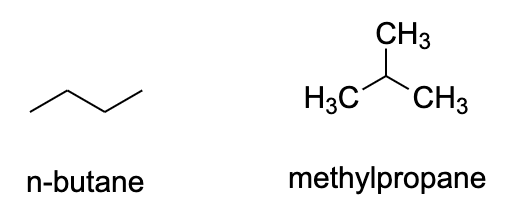
- We can see that in both of these structures, there are only carbon and hydrogen atoms and they can arrange themselves to different orientations in space.
- These are different types of structural isomerism and they are namely chain isomerism, position isomerism and functional isomerism.
- All these are exhibited by compounds having atoms of the same compound change and in functional group isomerism also the position of the various functional groups substituted in the chain change and we obtain functional isomers.
- In chain isomerism, the orientation of the chain differs in each of the isomers as the position of atoms differ in the chain.
Note:
Isomers are compounds that have the same molecular formula but different structural orientation. These compounds are of a variety of uses in the industry because when they orient in different arrangements, their properties may also differ.
