Question
Question: How are covalent bonds represented in Lewis dot diagrams?...
How are covalent bonds represented in Lewis dot diagrams?
Solution
For making Lewis dot structure of any molecule first we have to know about the atomic number and electronic configuration of each atom present in that molecule, because in that structure the dot shows the valence electrons of the outermost shell.
Complete answer:
In the Lewis dot structure we will display the valence electrons of the outermost shell in the form of dots on each atom, which are present in the molecule. And covalent bonds between two atoms will show by the simple bond or dots also.
Methane (CH4) and chlorine (Cl2) molecules are the examples of covalent molecules and their Lewis dot structure are shown as follow.
-In methane molecules, carbon is the central atom and in carbon four valence electrons are present and in hydrogen one valence electron is present. Four valence electrons of carbon will form four bonds with four hydrogen atoms and its Lewis dot structure is shown as follow:
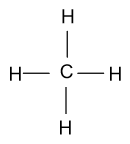
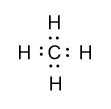
-Similarly in chlorine molecules, one covalent bond is present between two chlorine atoms and in the outermost shell of chlorine seven valence electrons are present. Lewis dot structure of chlorine molecule is shown as follow:
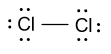
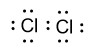
So, covalent bonds in Lewis dot structure may be shown by the single line or by dots also.
Note:
Here some of you during forming the Lewis dot structure may put two electrons in place of the bond on the both atoms which are present around that bond but that will be wrong. You have to put only two electrons between those two atoms in place of covalent bond.
