Question
Question: How are \[1 - \] nitro propane, \[2 - \]Nitro propane and \[2 - \]methyl-\[2 - \]-nitropropane disti...
How are 1− nitro propane, 2−Nitro propane and 2−methyl-2−-nitropropane distinguished from each other using the nitrous acid?
Solution
2−Nitropropane is a synthetic colourless liquid, flammable in nature and slightly soluble in water whereas greatly soluble in aromatic compounds like ether, hydrocarbons, esters, ketones. Its molecular formula isC3H7NO2.
Complete answer:
Now let’s move to each option one by one to see their reaction with nitrous acid, as they react with nitrous acid to produce coloured compounds.
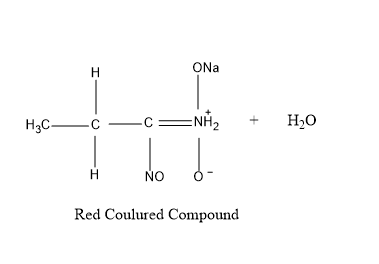
So , first taking 1− nitropropane – It reacts with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes , which further dissolves in alkali NaOH to produce red colored compound as shown in figure given below .
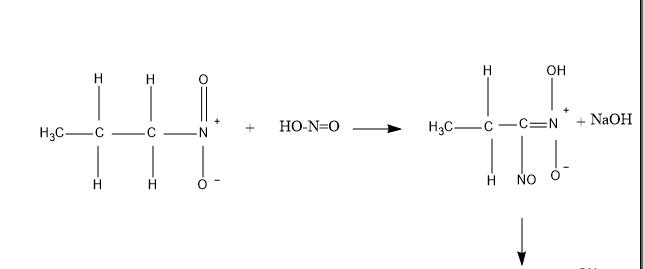
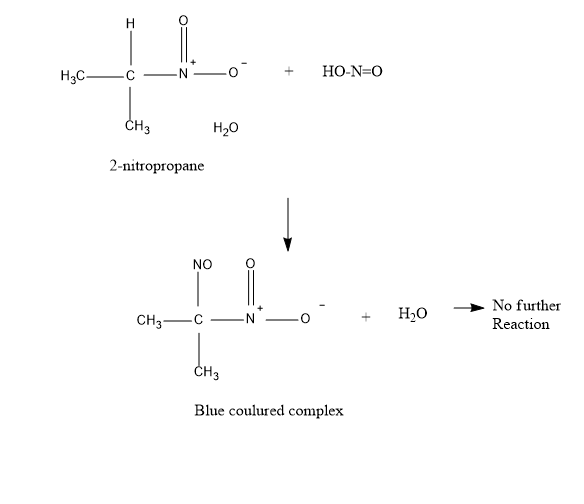
Now, talking about 2−Nitropropane – It also reacts with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes. But further it does not dissolve in alkali NaOH , because of the absence of α−H, (alpha hydrogen) .
Coming to 2−methyl-2−-nitropropane, it does not react even with nitrous acid to produce blue colored intermediate compound nitroso nitroalkanes because of the absence ofα−H, (alpha hydrogen).
So, here we are clear about the way to distinguish between 1− nitropropane, 2−Nitropropane and 2−methyl-2−-nitropropane.
Note:
1− nitropropane is used as starting material for the formation of other compounds especially in paint industries. 2− Nitro propane is used as a solvent or additive in inks, varnishes, polymers. It is used as a solvent and also used in pharmaceutical companies for the formation of compounds like chlorphentermine , teclozan , phentermine . Basically they have a great use in chemical industries also especially in paint based industries .
