Question
Question: How anisole react with acetyl chloride (\(C{{H}_{3}}COCl\)) in the presence of anhydrous \(FeC{{l}_{...
How anisole react with acetyl chloride (CH3COCl) in the presence of anhydrous FeCl3 or AlCl3 ? Write the chemical equation for the reaction.
Solution
This reaction is a type of an electrophilic aromatic substitution reaction. Aluminium chloride here will act as a lewis acid. Structure of the anisole is shown below.
Complete step by step solution:
So, first of all in this solution, we will get information about Friedel-Crafts reaction. We know that anisole is a benzene derivative.
- Friedel-Crafts reaction is an organic coupling reaction involving an electrophilic aromatic substitution that is used for the attachment of substituents to aromatic rings. There are two primary types of Friedel-Crafts reactions- the alkylation and acylation reactions.
- The reaction that is provided in the question is Friedel-Crafts acylation. Friedel-Crafts acylation reaction involves the addition of an acyl group to an aromatic ring. We will do it by using an acid chloride (R-COCl) and a Lewis acid catalyst such as AlCl3. In a Friedel-Crafts acylation reaction, the aromatic ring is transformed into an aryl ketone.
- So, from the above paragraph, we can say that anisole when reacts with acetyl chloride it will get transformed into ketone. Now, we will see the reaction of anisole into ketone.
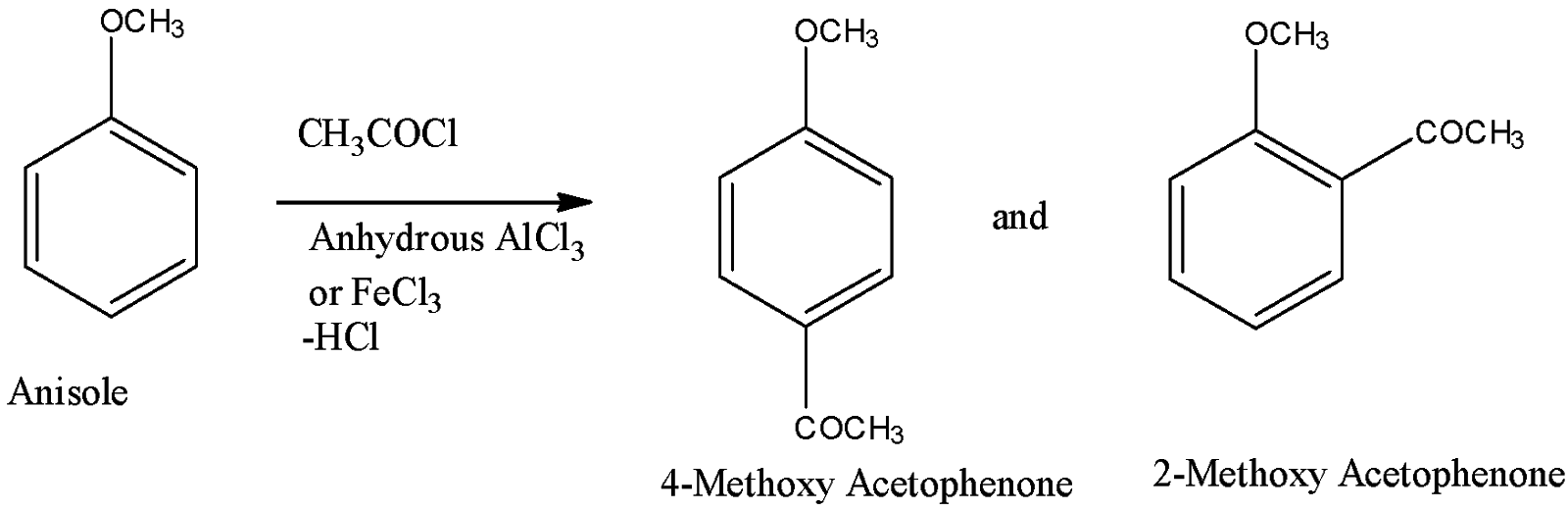
From the above figure, we got our answer and we can say that, when anisole reacts with acetyl chloride in the presence of anhydrous aluminium chloride as a catalyst, it will first generate an electrophile from acyl chloride which will get attacked by benzene ring to give a mixture of products. So, it will form 2-methoxy acetophenone and 4-methoxy acetophenone. Here, the methoxy group is an ortho-para director group so that we got a mixture of ortho and para products. This reaction is Friedel Crafts acylation reaction (an electrophilic aromatic substitution reaction).
Note: Remember that we cannot use any inorganic acid in place of lewis acid here in Friedal crafts’ reaction. Note that the alkoxy group is always an ortho-para director in electrophilic aromatic substitution reactions.
