Question
Question: Homolytic fission of a covalent bond leads to the formation of: (A) Electrophile (B) Nucleophile...
Homolytic fission of a covalent bond leads to the formation of:
(A) Electrophile
(B) Nucleophile
(C) Free radical
(D) Carbocation
Solution
Homolytic fission: It is defined as the cleavage in which a covalent bond breaks in such a way that each atom takes away one electron of the shared pair. They contain an unpaired electron. The bond is broken evenly in the homolytic fission.
Complete step-by-step answer:
Homolytic cleavage results in the generation of free radicals, which are neutral species (atoms or groups) that contain an unpaired electron. The bond is cleaved in such a way that each atom gets one electron each. We can say that the bond is cleaved evenly. Homolytic cleavage can be represented as follows;
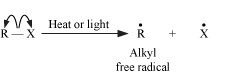
This cleavage often takes place in non-polar bonds and is favored by high temperatures, ultraviolet radiations, and by the presence of radical initiators such as peroxides.
Hence, the homolytic fission of a covalent bond leads to the formation of free radicals.
The correct option is C.
Additional Information:
Free radical means, a molecule or atom that contains an unpaired electron but is neither positively nor negatively charged. Free radicals are usually highly reactive and unstable.
Alkyl free radicals like carbocations are planar chemical species. In free radicals, the unhybridized p-orbital contains an odd electron.
Note: The possibility to make a mistake is that you may choose option D. In homolytic fission, free radicals are formed, not carbocations. Carbocations are formed in heterolytic fission in which the electrons of the covalent bonds are taken away by one of the bonded atoms.
