Question
Question: Here,\[E1cB\] Reaction is given by which of the following? A.\[C{F_3} - CHC{l_2}\] B.
C.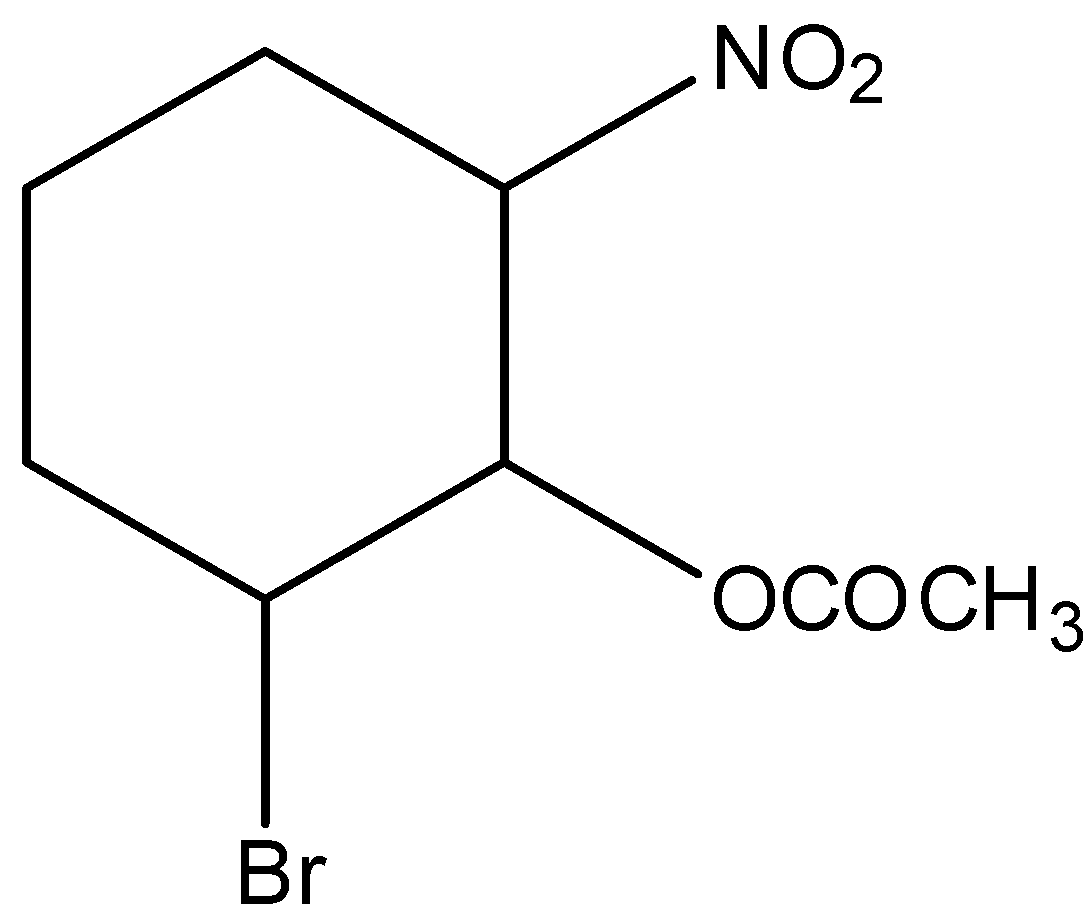
D.All of these
Solution
We must have to know the E1cB reaction otherwise it is known as elimination unimolecular conjugate base. Hence, this reaction is classified under the elimination reaction and this reaction is taking place under basic conditions with the poor leaving group and the hydrogen is removed which is comparatively acidic. And the first order of E1cB reaction which respects the conjugate base.
Complete answer:
Here, 2,2-dichloro-1,1,1-trifluoroethane undergoes the E1cB reaction. Because it contains a poor leaving group. Here in the first step, the C-H bond will break which is known as deprotonation. The C-H bond is relatively acidic. And secondly, the carbon-leaving group will break and there is a formation of C-C bond which is (pi) bond.
Here, the 2,2-dichloro-1,1,1-trifluoroethane reacted with base. And the base will attack the hydrogen which is present in the beta position. The leaving group is attached to the alpha position. During the deprotonation step, there is a formation of anion. Let’s see the reaction,
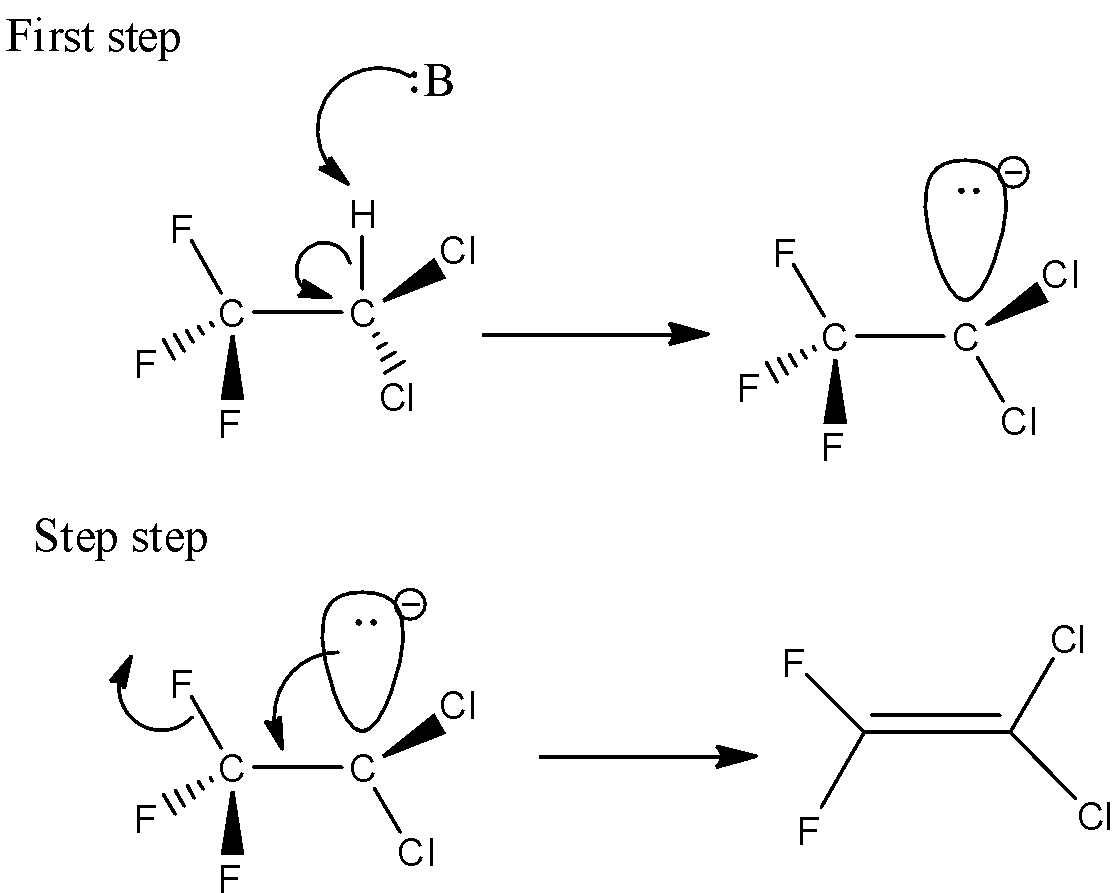
Hence, option (A) is correct.
Here, 1-bromo-2-nitrobutane does not occur in the E1cB reaction. Because, it does not contain a poor leaving group. Here, the bromine is attached at the beta position. Thus, it should act as a leaving group. But the bromine is not a poor leaving group. Thus, E1cB reaction does not take place in 1-bromo-2-nitrobutane. Hence, the option (B) is incorrect.
In 2-bromo-6-nitro cyclohexyl acetate gives the E1cB reaction. Because, here the nitrogen group is act as leaving group and the reaction can be written as,
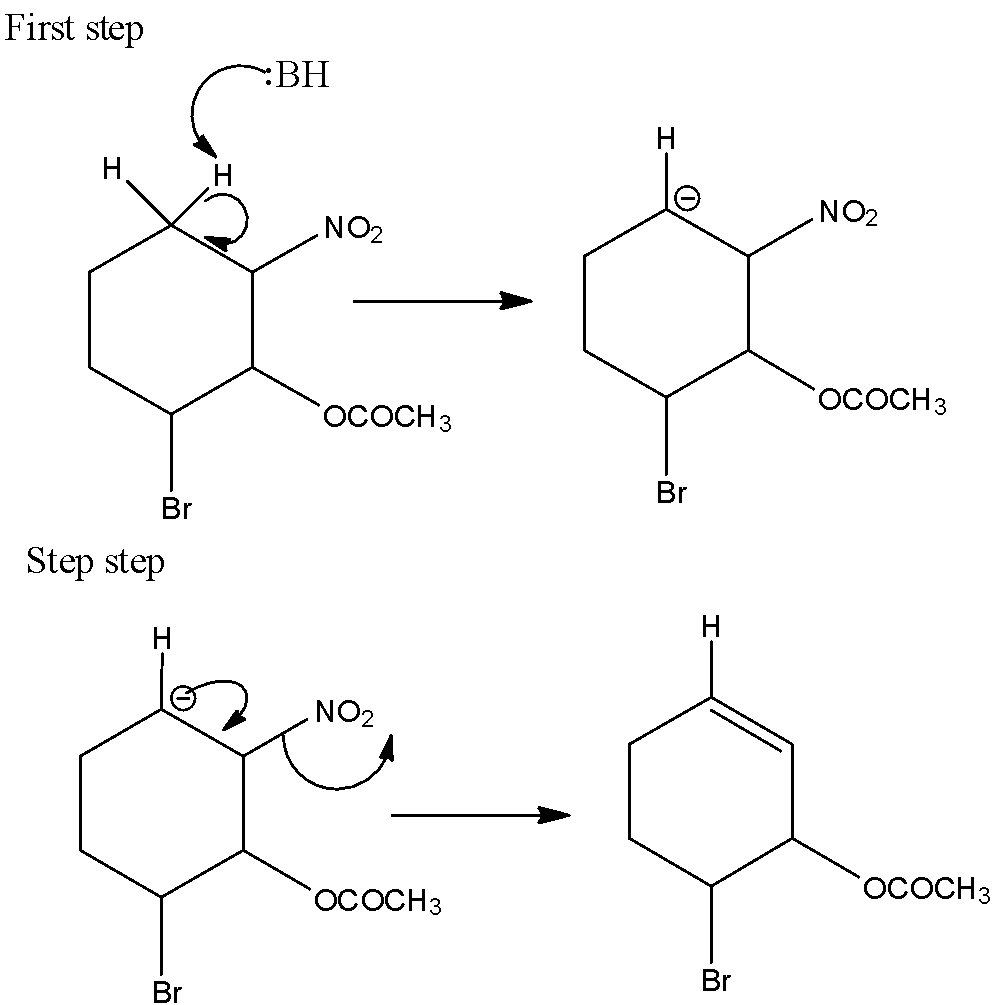
Hence, option (C) is correct.
Among this option, only 2, 2-dichloro-1, 1, 1-trifluoroethane and 2-bromo-6-nitro cyclohexyl acetate undergoes E1cB reaction. Hence, the option (D) is incorrect.
Note:
We have to know that the E1cB reaction takes place in two steps. In the first step, the deprotonation will occur and there is a formation of anion. Here, the hydrogen is attached to the alpha carbon atom and the C-H bond is more acidic. In second place there is a displacement if the leaving group will take place and form C-C pi bind by the breakage of carbon – leaving group bond.
