Question
Question: Here (A), (B), (C), (D), (E), (F), (G) and (H) are as follows. (A) : \({{(N{{H}_{4}})}_{2}}C{{r}_...
Here (A), (B), (C), (D), (E), (F), (G) and (H) are as follows.
(A) : (NH4)2Cr2O7 (B): NH3 (C) : Cr2O3 (D) : N2 (E) : AlN (F) : CrCl3 (G) : Na2CrO4
(H) : PbCrO4
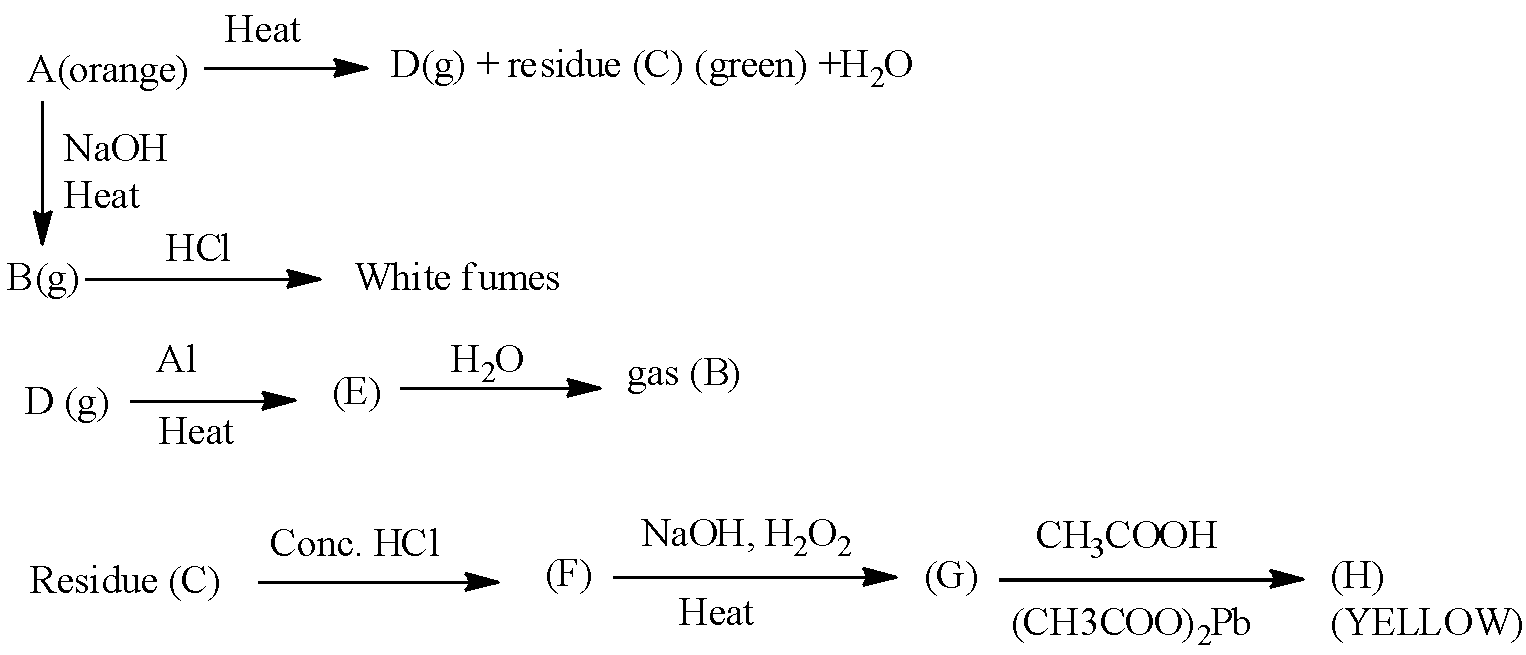
Write the above chemical reaction.
Solution
Ammonium chromate is an orange color compound and it is easily going to form nitrogen gas while on heating. Means ammonium chromate compound is heat sensitive in nature. While treated with a base like sodium hydroxide ammonium chromate is going to release ammonia gas as a product.
Complete answer:
- In the question it is asked to write the chemical reaction by following the given chemical route.
- First we have to start writing the chemical reaction with ammonium chromate (A).
- The chemical ammonium chromate (A) is going to react with sodium hydroxide.
A(NH4)2Cr2O7+2NaOHΔB2NH3+2H2O+Na2O+2CrO3
- On heating the compound ‘A’ with sodium hydroxide ammonia gas (B) is going to form. In the above chemical reaction we can see that one mole of A reacts with 2 moles of sodium hydroxide and forms 2 moles of ammonia gas (B) as the product.
- The compound B is going to react with hydrochloric acid and forms white fumes due to the liberation of the ammonium chloride gas. The chemical reaction is as follows.
NH3+HCl→NH4Cl
- Now coming to the formation of the compound D from A.
- The compound ‘A’ on heating produces nitrogen gas. With nitrogen there is a formation of the green color residue called chromium oxide. The chemical reaction is as follows.
(NH4)2Cr2O7ΔDN2+CCr2O3+4H2O
- The nitrogen gas (D) which is formed in the above chemical reaction is going to react with aluminum and forms aluminum nitride chemical as ‘E’. The chemical reaction is as follows.
2Al+DN2heat2EAlN
- The compound ‘E’ on reaction with water (hydrolysis) forms aluminum hydroxide and ammonia gas (B) as the products. The chemical reaction is as follows.
AlN+3H2O→Al(OH)3+BNH3
- The chemical chromium trioxide (C) which is formed from ‘A’ is going to react with hydrochloric acid and forms chromium chloride (F) as the product. The chemical reaction is as follows.
Cr2O3+6HCl→F2CrCl3+3H2O
- The chemical ‘F’ on heating with sodium hydroxide and hydrogen peroxide forms a compound called sodium chromate (G). The chemical reaction is as follows.
2CrCl3+4NaOH+2H2O2heatG2Na2CrO4+6HCl+H2
- The compound (G) which is formed in the above chemical reaction is going to react with acetic acid and forms a yellow precipitate called lead chromate (H). The chemical reaction is as follows.
Na2CrO4+(CH3COOH)2PbCH3COOHH,yellow pptPbCrO4+2CH3COONa
Note:
We have to write the above chemical reactions very carefully because if we are going to write one chemical reaction wrong automatically the total chain reactions will get disturbed and they are not going to be balanced also.
