Question
Question: Helium gas goes through a cycle ABCDA (consisting of two isochoric and isobaric lines) as shown in f...
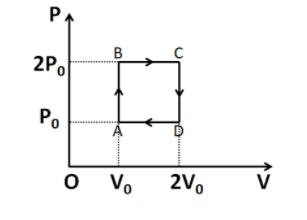
Helium gas goes through a cycle ABCDA (consisting of two isochoric and isobaric lines) as shown in figure. Efficiency of this cycle is nearly (assume the gas to be close to ideal gas)
(A) 15.4%
(B) 9.1%
(C) 10.5%
(D) 12.5%
Solution
The efficiency of a cycle is equal to the ratio of the work done in that cycle to the heat absorbed. The work done can be easily evaluated by calculating the area of the P-V diagram given in the question.
Complete step-by-step solution:
We know that the work done in a process is equal to the area of the P-V diagram. So the work done in the given cycle is given by
W=Ar(ABCD)
⇒W=AB×AD
From the given diagram, we have AB=P0 and AD=V0. Substituting these above, we get
W=P0V0.....................(1)
Since the direction of the loop is clockwise, so the work done is positive.
Now, the heat is absorbed in the processes AB and BC. These two respectively are isochoric and isobaric processes. So we have
QAB=nCVΔT
We know that CV=2fR. So we have
QAB=2fnRΔT
⇒QAB=2fVΔP (1) (from the ideal gas equation for constant pressure)
For the process AB, we have
ΔP=2P0−P0
⇒ΔP=P0
Also V=V0
Substituting these in (1) we get
QAB=2fP0V0....................(2)
Now, for the process BC, we have
QBC=nCPΔT
We know that CP=(2f+1)R. So we have
QBC=(2f+1)nRΔT
⇒QBC=(2f+1)PΔV................(3) (from the ideal gas equation for constant pressure)
For the process BC we have
ΔV=2V0−V0
⇒ΔV=V0
Also P=2P0
Substituting these in (3) we get
QBC=2(2f+1)P0V0....................(4)
Adding (2) and (4) we get the total heat absorbed as
Q=2fP0V0+2(2f+1)P0V0
⇒Q=P0V0[2f+2(2f+1)]
Now, since Helium is a monatomic gas, we have f=3. Substituting this above, we get
Q=P0V0[23+2(23+1)]
⇒Q=213P0V0......................(5)
Now, the efficiency is given by
η=QW
Putting (1) and (5) we get
η=213P0V0P0V0
⇒η=132≈0.154
Multiplying by 100, we get the percentage efficiency of the cycle equal to 15.4%.
Hence, the correct answer is option (1).
Note: The sign of the work done is very much important. It can be easily found out by using the direction of the loop of the cycle in a P-V diagram. A clockwise loop means a positive work, while an anticlockwise loop means a negative work.
