Question
Question: Heating of carboxylic acid with soda lime results in: A. dehydration B. dehydrogenation C. dec...
Heating of carboxylic acid with soda lime results in:
A. dehydration
B. dehydrogenation
C. decarboxylation
D. addition of O2
Solution
Carboxylic acid is an organic compound having −COOH group. The chemical formula of soda lime is CaHNaO2. We know that the soda lime is obtained when calcium oxide is mixed with sodium hydroxide.
Complete step by step answer:
Soda lime is used in respiration. It eliminates carbon dioxide and prevents carbon dioxide poisoning. It is used as a drying agent. It absorbs carbon dioxide.
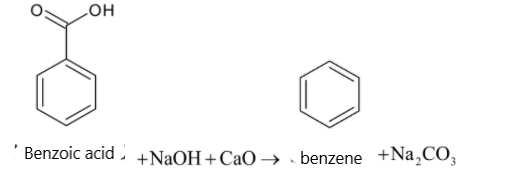
Soda lime is the mixture of NaOH and CaO. But it is written as sodium hydroxide in equations. Sodium hydroxide removes CO2 in a carboxylic acid molecule which produces alkane, i.e benzene. And the CO2 reacts with sodium hydroxide to give sodium carbonate. The function of CaO is that it makes NaOH less reactive.
Decarboxylation reaction is a chemical reaction in which carbon dioxide is removed from any compound. While carboxylation is the addition of carbon dioxide to any compound. Decarboxylation reaction is possible only if there is a carboxyl group in the compound.
When a carboxylic acid is heated with soda lime, it undergoes decarboxylation. The carboxyl group in carboxylic acid is converted to sodium carbonate. It also forms a hydrocarbon. The chemical reaction is given below:
RCOOH+NaOHCaOΔRH+Na2CO3
Carboxylic acid Hydrocarbon
So when carboxylic acid is heated with soda lime, the reaction is known as decarboxylation.
So, the correct answer is Option C.
Note: Sodium salts of carboxylic acids can also undergo decarboxylation reaction by heating them with soda lime. This also gives the same product as carboxylic acids. Let’s take an example. When sodium benzoate is heated with soda lime, it produces benzene, sodium carbonate and water molecules. The reaction is given below: