Question
Question: Heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives X as the major product. Reaction of X with $...
Heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives X as the major product. Reaction of X with Hg(OAc)2/H2O followed by NaBH4 gives Y as the major product. Y is
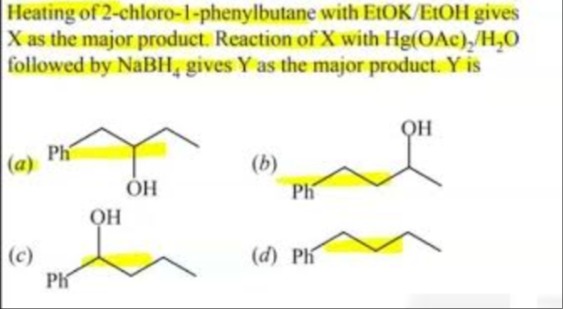
1-phenylbutan-2-ol
2-phenylbutan-1-ol
1-phenylbutan-1-ol
2-phenylbutan-2-ol
1-phenylbutan-1-ol
Solution
The reaction begins with 2-chloro-1-phenylbutane (Ph-CH2-CH(Cl)-CH2-CH3). Treatment with a strong base like EtOK/EtOH at elevated temperatures promotes an E2 elimination reaction. The possible beta-hydrogens are on C1 (adjacent to the phenyl group) and C3.
-
Formation of X (Alkene):
- Elimination of a beta-hydrogen from C1 and the chlorine from C2 yields Ph-CH=CH-CH2-CH3 (1-phenylbut-1-ene).
- Elimination of a beta-hydrogen from C3 and the chlorine from C2 yields Ph-CH2-CH=CH-CH3 (1-phenylbut-2-ene). According to Zaitsev's rule, the more substituted alkene is favored. However, both are disubstituted. The alkene conjugated with the phenyl ring (1-phenylbut-1-ene) is more stable. Thus, X is predominantly 1-phenylbut-1-ene.
-
Formation of Y (Alcohol) via Oxymercuration-Demercuration: The reaction of alkene X with Hg(OAc)2/H2O followed by NaBH4 is oxymercuration-demercuration, a method for hydrating alkenes. This reaction follows Markovnikov's rule, where the hydroxyl group adds to the more substituted carbon of the double bond, and carbocation rearrangements do not occur.
For X = Ph-CH=CH-CH2-CH3:
- The double bond is between C1 (attached to Ph) and C2 (attached to CH2CH3).
- C1, being directly attached to the phenyl ring, is considered more substituted in this context for Markovnikov addition.
- Therefore, the -OH group adds to C1, and a hydrogen atom adds to C2.
- The intermediate after oxymercuration is Ph-CH(OH)-CH(HgOAc)-CH2-CH3.
- Demercuration with NaBH4 replaces the -HgOAc group with a hydrogen atom.
- The final product Y is Ph-CH(OH)-CH2-CH2-CH3, which is 1-phenylbutan-1-ol.
The major product Y is 1-phenylbutan-1-ol.
