Question
Question: Heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives X as the major product. Reaction of X with \...
Heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives X as the major product. Reaction of X with Hg(OAc)2/H2O followed by NaBH4, gives Y as the major product. Y is:
(a)
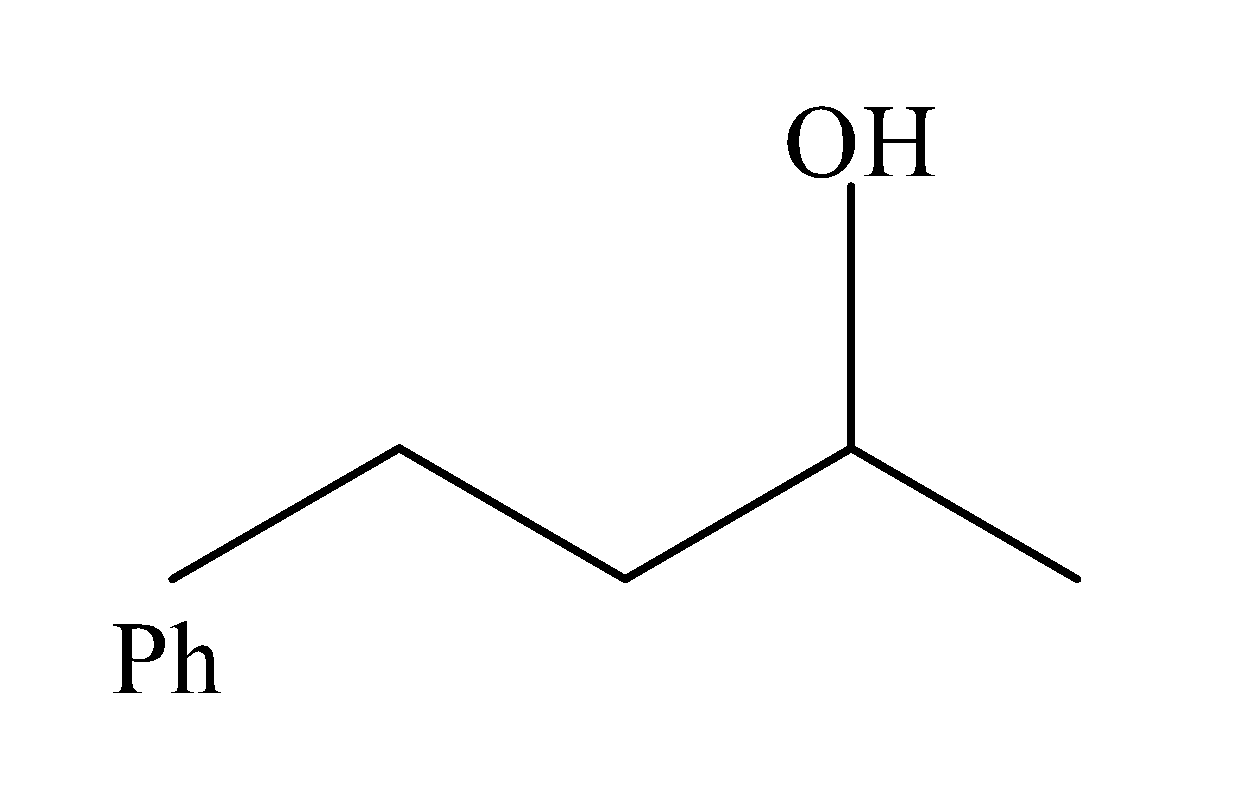
(b)
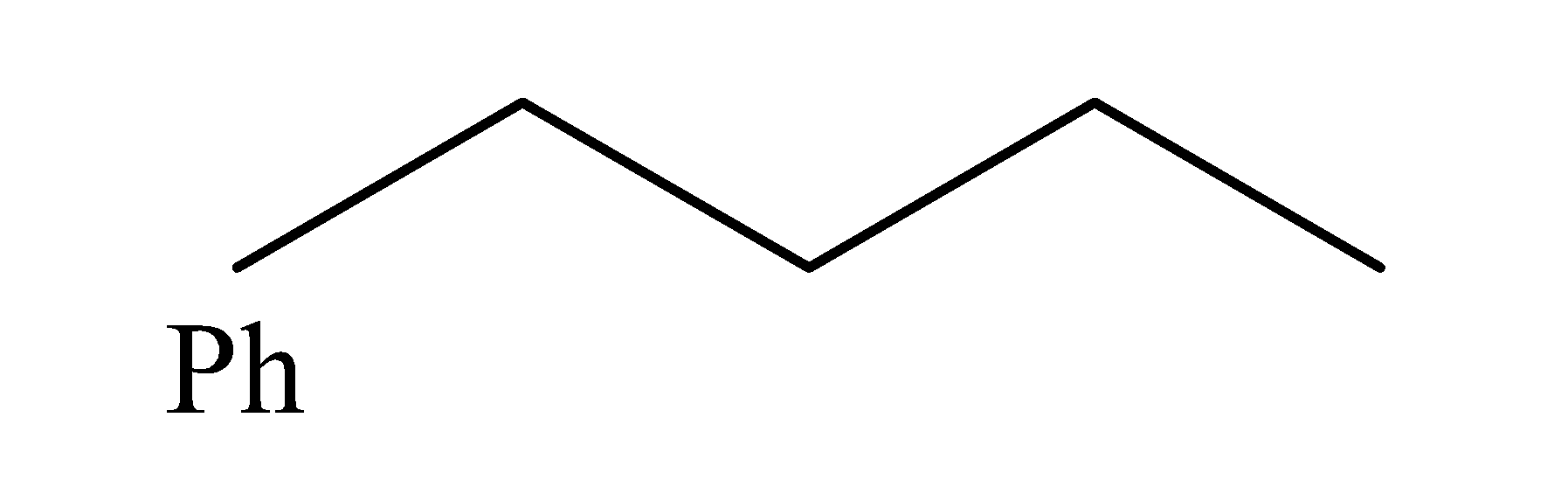
(c)
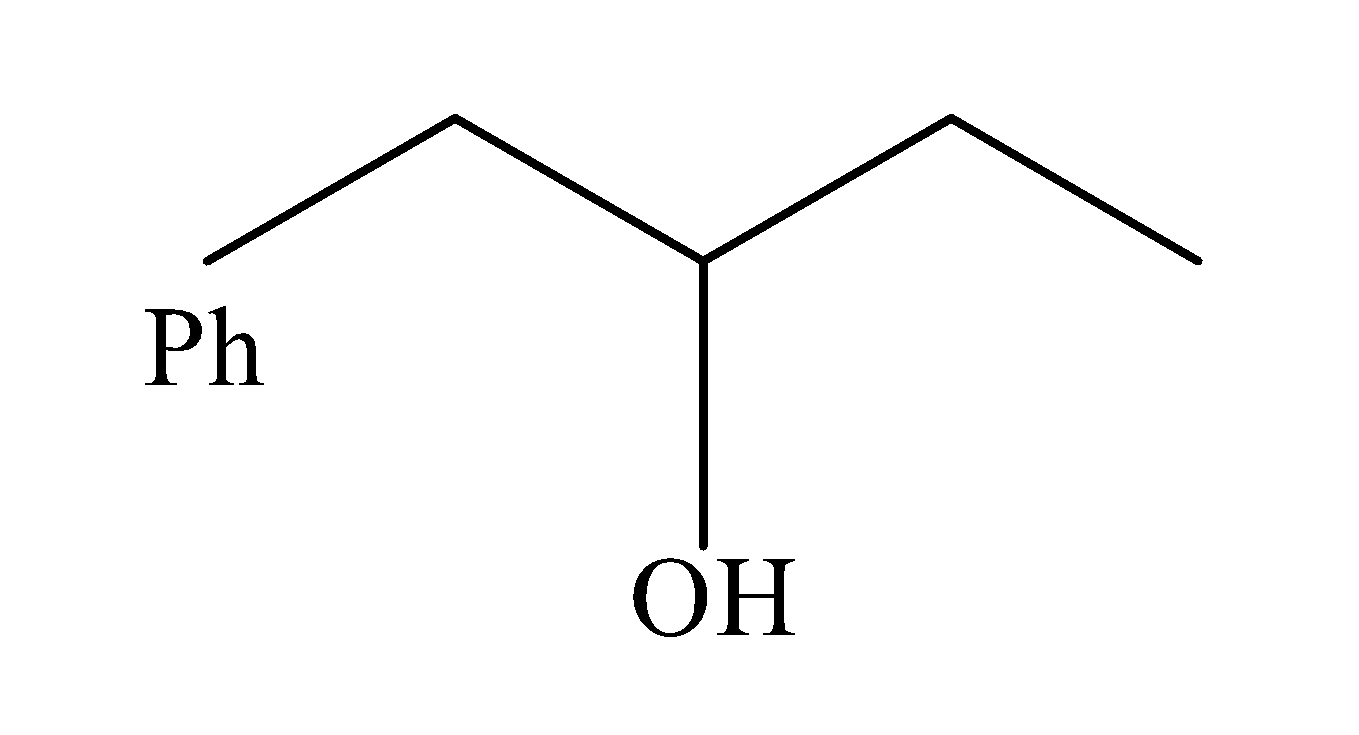
(d)
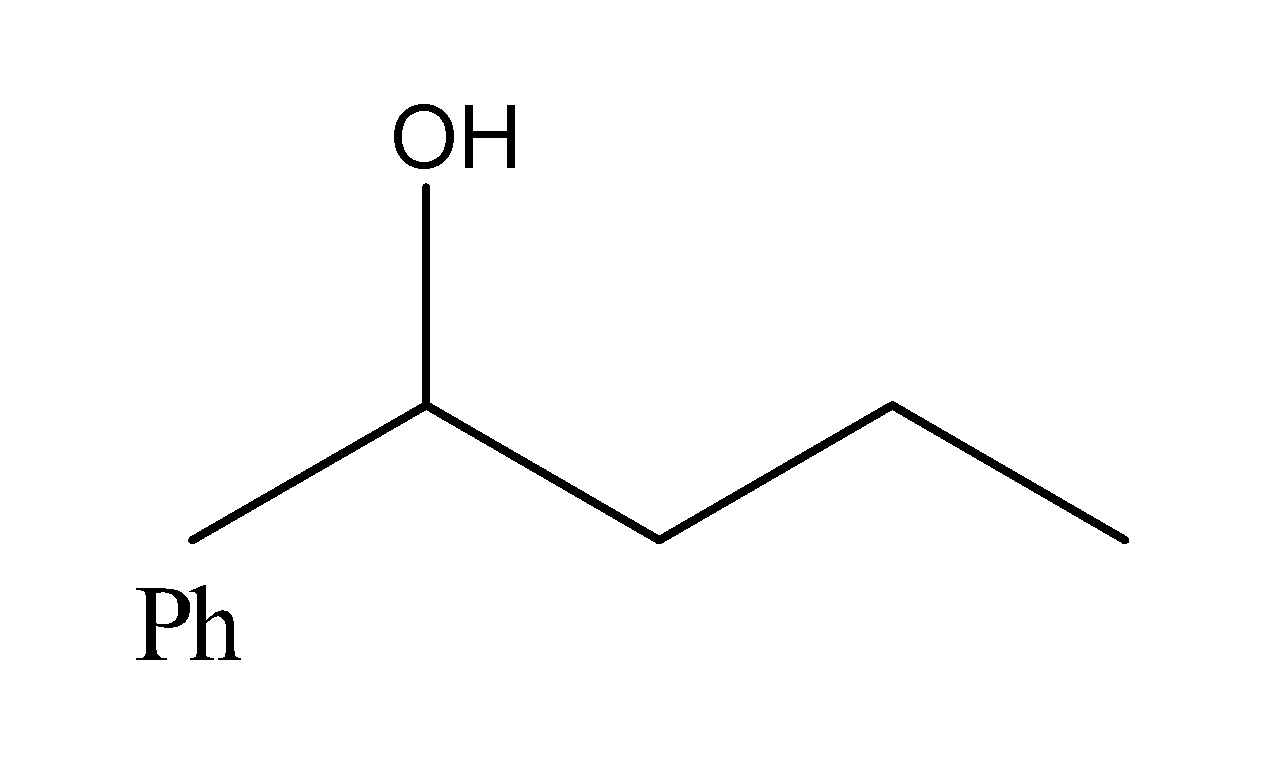
Solution
. When 2-chloro-1-phenylbutane is heated with the potassium ethoxide in the presence of ethanol it undergoes elimination reaction and results in the formation of double bond and when this double bond compound is treated with Hg(OAc)2/H2O followed by NaBH4, it undergoes oxymercuration-demercuration reaction resulting in the formation of compound which contains the functional group -OH. Now solve the reaction.
Complete step by step answer:
2-chloro-1-phenylbutane consists of a phenyl ring and butane with chlorine attached to carbon number 2 in butane. It is an organic compound.
EtOK is potassium ethylene oxide and it is colorless gas and is liquid at low temperatures and is highly flammable i.e. that catches fire easily and undergoes alkylation reactions with many organic compounds and EtOH is the ethanol.
2-chloro-1-phenylbutane when is heated with potassium ethylene oxide and ethanol, it undergoes elimination reaction and it results in the formation of an alkene as the major product and the chlorine is removed from 2-chloro-1-phenylbutane. Elimination reactions are those reactions in which the two substituents are removed i.e. the carbon- hydrogen and carbon-halogen bonds break to form a double bond and, in these reactions, the major product formed is the alkene which is the most stable. The reaction occurs as:
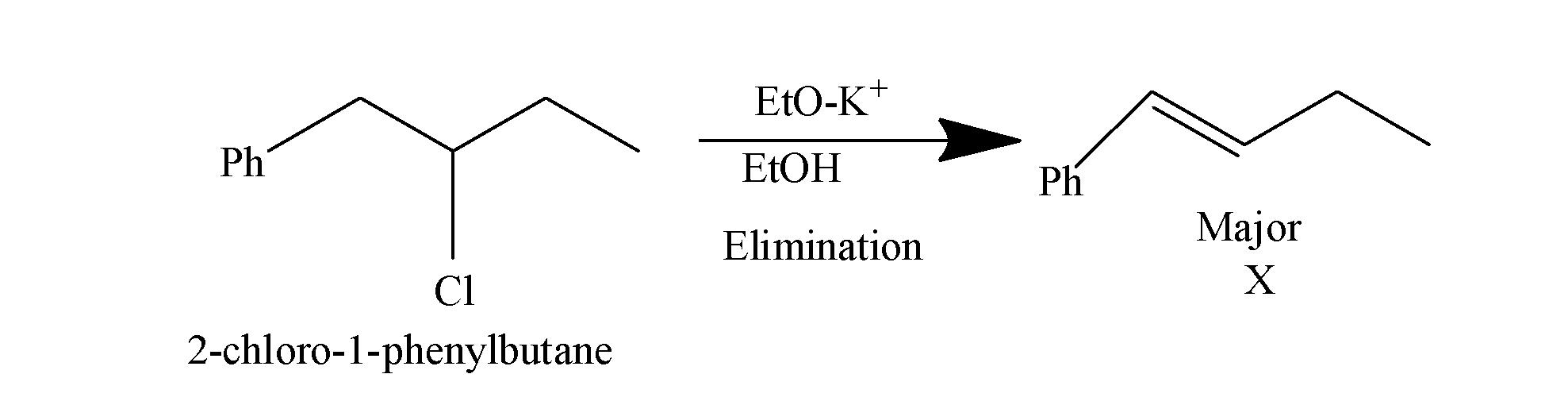
Now this, alkene so formed when treated with mercury acetate : Hg(OAc)2/H2O followed by NaBH4 i.e. undergoes oxymercuration-demercuration reactions and results in the formation of alcohol as the major product(Y). The reaction occurs as:
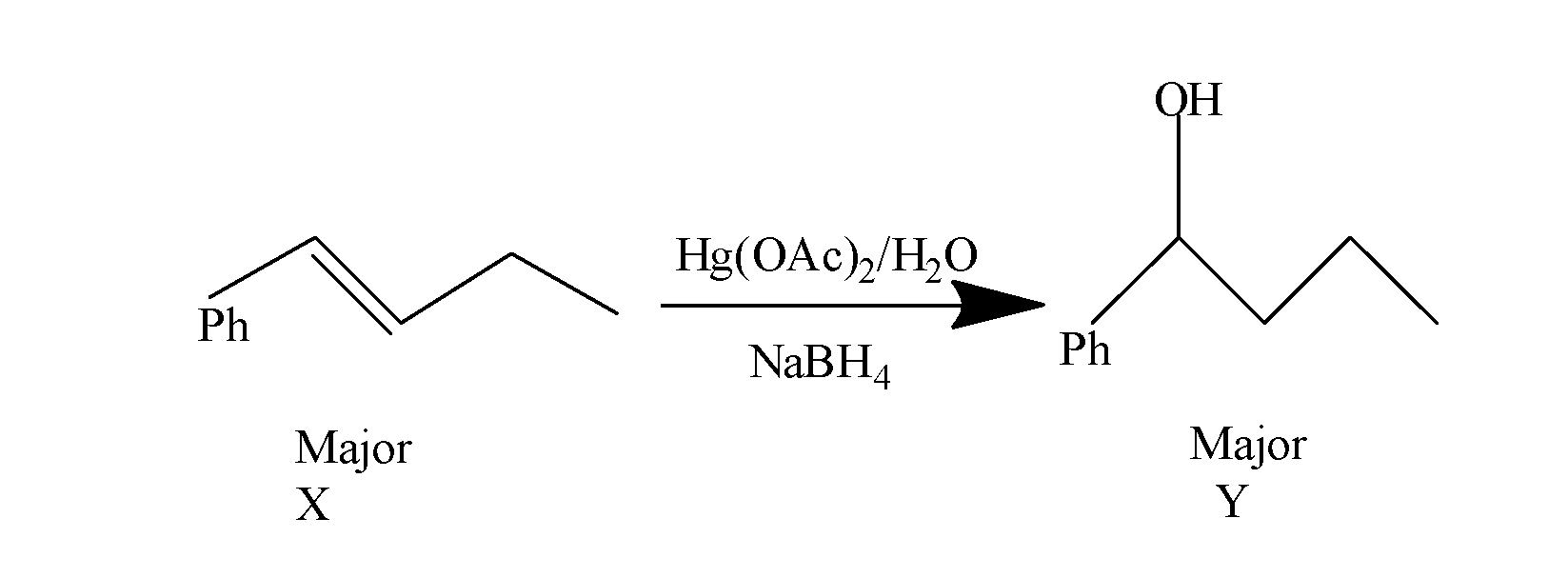
Hence, the heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives alkene as the major product. Reaction of alkene with Hg(OAc)2/H2O followed by NaBH4, gives alcohol as the major product. So, the correct answer is “Option D”.
Note: Ethylene oxide is an organic compound, also known as the epoxide as it is cyclic ether and consists of one oxygen and three carbon atoms in a c cyclic ring and it reacts with potassium forming potassium ethoxide and can act as both oxidizing as well as reducing agents.
