Question
Question: he intramolecular hydrogen bonding is present in: A. o-nitrophenol B. m-nitrophenol C. p-nitro...
he intramolecular hydrogen bonding is present in:
A. o-nitrophenol
B. m-nitrophenol
C. p-nitrophenol
D. Phenol
Solution
Intramolecular hydrogen bonding is formed within a compound when there is a high electronegative element present along with neighboring hydrogen atoms surrounding it such that there exist partial force of attraction due to formation of oppositely charged partial charges.
Complete answer:
The o-nitrophenol is a compound in which there is a nitro group present at the benzene and corresponding to it, there lies a hydroxyl group at the ortho position with respect to the nitro group. There exists an intramolecular hydrogen bonding in o-nitrophenol. Let us understand this from the diagram of o-nitrophenol.
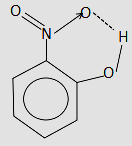
In the above structure, we can see that the hydroxyl group is attached to the nitrobenzene at the ortho position. The arrow shown between the nitrogen and oxygen atom shows the dative/coordinate bond in which there is a complete transfer of electrons from the nitrogen atom to the vacant orbital of oxygen atom. Now coming to the intramolecular hydrogen bonding, the dotted line between oxygen and hydrogen is the intramolecular hydrogen bond that is present between the oxygen and hydrogen of the same compound. This provides an extra stability to the compound. There exists another form of hydrogen bonding which is known as intermolecular hydrogen bonding in which there is a hydrogen bond present between the electronegative atom of one compound and the hydrogen atom of another compound.
So, the correct answer is Option A.
Note:
The hydrogen bond is a weak intermolecular force of attraction that provides an extra stability to a compound and also helps to increase the stability of a compound dissolved in a polar solvent such as water through the formation of extra bonds.
