Question
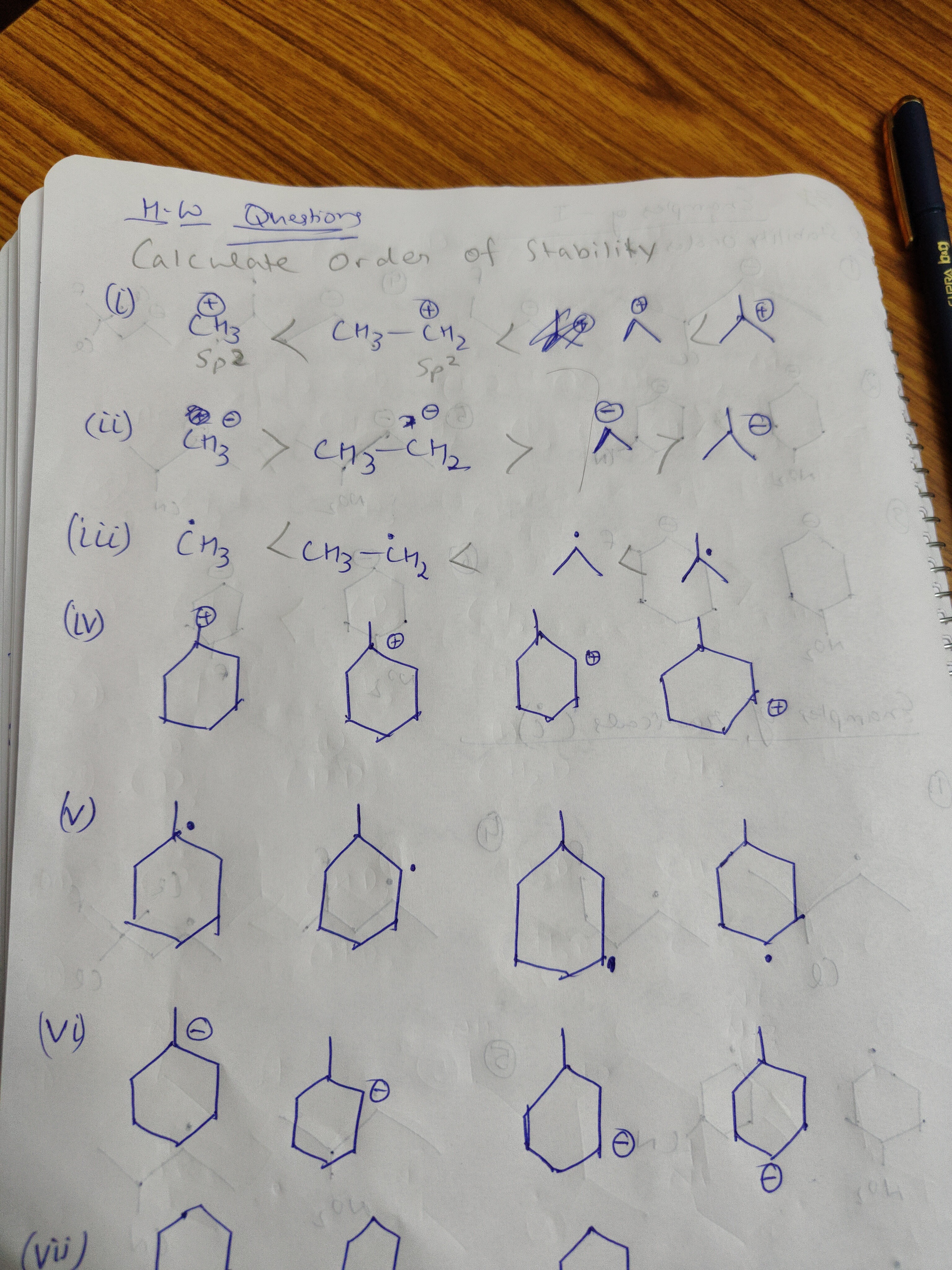
Question: H-W Questions Calculate Order of Stability (i) $CH_3^+$ < $CH_3CH_2^+$ < < $Sp^2$ $Sp^2$ (ii) $CH_...
H-W Questions Calculate Order of Stability
(i) CH3+ < CH3CH2+ < < Sp2 Sp2
(ii) CH3− > CH3CH2− > >
(iii) CH3. < CH3CH2. < <
(iv) (v) (vi) (vii)
Primary carbocation < Cyclohexyl (methyl para) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl adjacent)
CH3− < CH3CH2− < Cyclohexyl carbanion (methyl adjacent to charge) < Cyclohexyl carbanion (methyl meta to charge)
CH3. < CH3CH2. < Cyclohexyl radical (methyl meta to radical) < Cyclohexyl radical (methyl adjacent to radical)
Primary < Cyclohexyl (methyl para) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl adjacent)
Primary radical < Cyclohexyl (methyl para) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl adjacent)
Cyclohexyl (methyl adjacent) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl para) < Primary
Homoallylic (furthest) < Homoallylic (further) < Homoallylic (closer) < Allylic
Primary carbocation < Cyclohexyl (methyl para) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl adjacent); CH3− < CH3CH2− < Cyclohexyl carbanion (methyl adjacent to charge) < Cyclohexyl carbanion (methyl meta to charge); CH3. < CH3CH2. < Cyclohexyl radical (methyl meta to radical) < Cyclohexyl radical (methyl adjacent to radical); Primary < Cyclohexyl (methyl para) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl adjacent); Primary radical < Cyclohexyl (methyl para) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl adjacent); Cyclohexyl (methyl adjacent) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl para) < Primary; Homoallylic (furthest) < Homoallylic (further) < Homoallylic (closer) < Allylic
Solution
The stability of carbocations, carbanions, and free radicals is governed by inductive effects, hyperconjugation, resonance, and hybridization.
- Carbocations (+): Stability is enhanced by electron-donating groups (inductive effect, hyperconjugation) and resonance. The order of stability for alkyl carbocations is Tertiary > Secondary > Primary.
- Carbanions (-): Stability increases with electron-withdrawing groups. Alkyl groups, being electron-donating, destabilize carbanions. For hybridization, the order of stability is sp>sp2>sp3. For alkyl carbanions, the order is Methyl < Ethyl < Isopropyl < Tert-butyl.
- Free Radicals (.): Stability follows a similar trend to carbocations, increasing with electron-donating groups and resonance. The order is Tertiary > Secondary > Primary.
- Resonance: Allylic and benzylic species (carbocations, carbanions, radicals) are significantly stabilized by resonance.
- Cyclic Systems: Ring strain and the relative position of substituents to the charged/radical center influence stability.
Detailed breakdown for each part:
(i) Carbocations: The initial part shows CH3+ < CH3CH2+. The cyclic species are cyclohexyl carbocations with methyl substituents. The order of stability is: Primary carbocation (charge on the methyl carbon) < Cyclohexyl (methyl para to charge) < Cyclohexyl (methyl meta to charge) < Cyclohexyl (methyl adjacent to charge).
(ii) Carbanions: The provided inequality CH3− > CH3CH2− is incorrect because alkyl groups destabilize carbanions. The correct order of stability for the species shown is: CH3− < CH3CH2− < Cyclohexyl carbanion (methyl adjacent to charge) < Cyclohexyl carbanion (methyl meta to charge). The destabilizing effect of the methyl group increases as it gets closer to the carbanion center.
(iii) Free Radicals: The initial part shows CH3. < CH3CH2. . The cyclic species are cyclohexyl radicals with methyl substituents. The order of stability is: CH3. < CH3CH2. < Cyclohexyl radical (methyl meta to radical) < Cyclohexyl radical (methyl adjacent to radical). Alkyl groups stabilize radicals, with greater stabilization occurring when the group is closer to the radical center.
(iv) Carbocations (Cyclic): The order of stability for the cyclohexyl carbocations with a methyl substituent is: Primary (charge on methyl carbon) < Cyclohexyl (methyl para) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl adjacent).
(v) Free Radicals (Cyclic): The order of stability for the cyclohexyl radicals with a methyl substituent is: Primary (radical on methyl carbon) < Cyclohexyl (methyl para) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl adjacent).
(vi) Carbanions (Cyclic): The order of stability for the cyclohexyl carbanions with a methyl substituent is: Cyclohexyl (methyl adjacent) < Cyclohexyl (methyl meta) < Cyclohexyl (methyl para) < Primary (anion on methyl carbon). The methyl group destabilizes the carbanion, and this effect is strongest when the methyl group is adjacent to the charge.
(vii) Carbocations (Cyclic with double bonds): The species are cyclohexenyl carbocations. The order of stability, from least to most stable, is determined by the proximity of the double bond to the carbocation center, which allows for resonance stabilization: Homoallylic (double bond furthest) < Homoallylic (double bond further) < Homoallylic (double bond closer) < Allylic (direct resonance).
