Question
Question: \(H_{ 2 }S_{ 2 }O_{ 8 }\) and \(H_{ 2 }SO_{ 5 }\) both have a +6 oxidation state in sulfur. It is du...
H2S2O8 and H2SO5 both have a +6 oxidation state in sulfur. It is due to the:
A. Presence of peroxy group
B. Presence of superoxo group
C. Presence of a neutral group
D. Presence of ozone
Solution
Hint: It’s easy to know the answer to this question once you draw the structure of both of these compounds. Here in these structures, you will find which group is responsible for this +6 oxidation state of sulfur.
Complete step by step answer:
First, let’s know a little about the compounds given,
Peroxydisulfuric acid is the inorganic compound with the chemical formula H2S2O8. It is also called Marshall's acid.
Peroxymonosulfuric acid, (H2SO5), is also known as peroxysulfuric acid, or Caro's acid.
In both of these compounds, peroxy linkage is present due to which oxidation number of S comes out to be +6
We can understand this statement by the following discussion,
The oxidation number of peroxymonosulfuric acid (H2SO5) is -
2 + x -10 = 0, where x is the oxidation number of sulfur
So, x = +8
However, this cannot be true, as the oxidation number of S cannot exceed the +6 oxidation state.
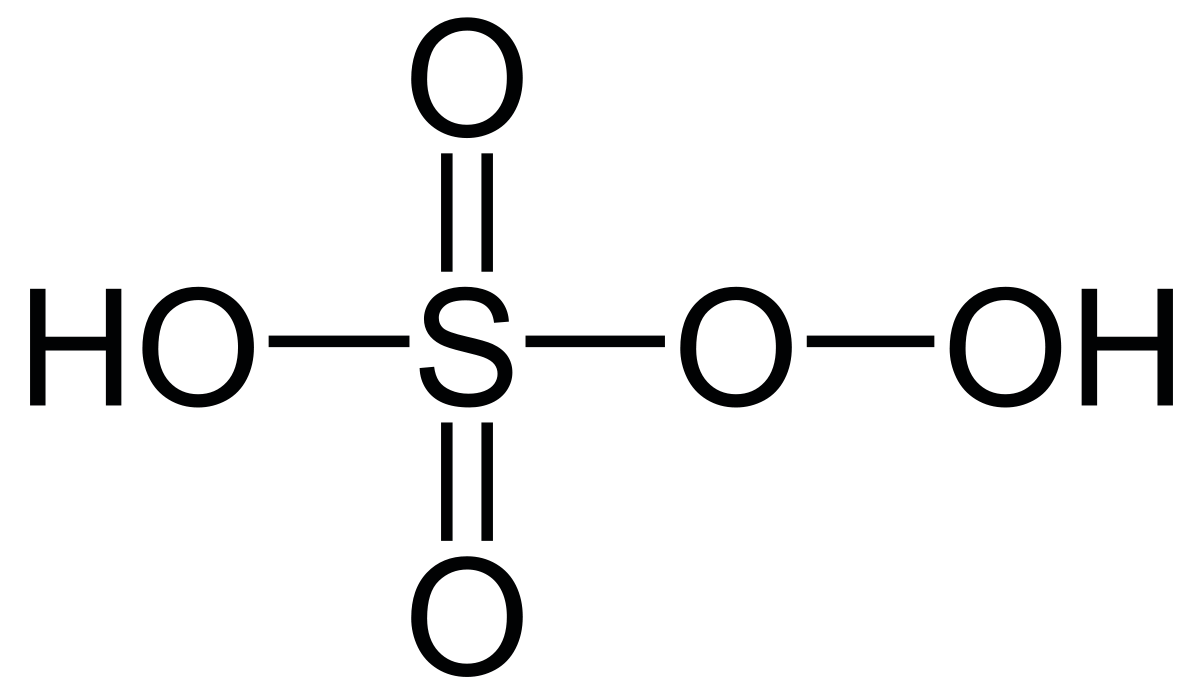
This odd oxidation number of sulfur is due to the peroxy linkages involving S in H2SO5,
So, oxidation number of S in H2SO5 is,
2(+1) + x + 3(-2) + 2(-1) = 0 (for peroxy linkage)
Hence, x = +6.
So the oxidation number of S in H2SO5 is +6.
Now, in Marshalls acid, which is peroxydisulfuric acid, H2S2O8,

oxidation state of S is -
2(+1) + 2x + 6(-2) + 2(-1) = 0
So, x = +6.
That is the oxidation numbers of both S in Marshall’s acid are +6.
Therefore, we can conclude that the correct answer to this question is option A.
Note: We should also know that there other oxo acids of sulfur that have been characterized contain a variety of structural features. Few of them are -
1. They have tetrahedral sulfur coordinated to oxygen.
2. They have terminal and bridging oxygen atoms.
3. They have terminal peroxo groups and terminal S=S
4. They have chains of (−S−)n
