Question
Question: \( {H_2}{S_2}{O_8} \) and \( {H_2}S{O_5} \) both have \( + 6 \) oxidation state of Sulphur. It is du...
H2S2O8 and H2SO5 both have +6 oxidation state of Sulphur. It is due to the:
(A) Presence of peroxy group
(B) Presence of superoxo group
(C) Presence of neutral group
(D) Presence of ozone
Solution
If we know the structure of H2S2O8 and H2SO5 we can see and check which of the following group is present in these compounds. Also, peroxy group is O22− , superoxo group is O2− , neutral can be O2 and ozone is O3 .
Complete Step by step answer:
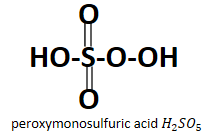
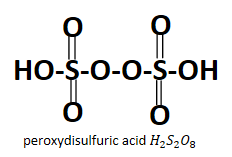
Looking at the structure it is obvious that both the acids have peroxo group attached to them which is O22− , in case of peroxydisulfuric acid it is present in between the two sulfur atoms whereas in case of peroxymonosulfuric acid it is the −O−OH peroxy group which is linked to the sulfur atom. Also it is very obvious from their names that they have a peroxy group contained in them.
So option (A) is correct.
Additional information:
The oxidation state of the sulfur atom could be calculated assuming all the bonds as ionic and when the bond between sulfur and oxygen is broken then the more electronegative atom which is here is oxygen will carry one negative charge so if again the double bond will be broken then we will have O2− left and there will be +2 charge on sulfur atom. And hence it can be confirmed that sulfur will carry +6 charge in both the compounds. Also it is not always the case but is valid for sulfur and phosphorus compounds.
Note:
The difference between the peroxo, superoxo, oxo and ozone should be carefully noted as they have a very slight difference between them and might be confusing sometime. Also while finding the oxidation state you must know the structure of the compound.
