Question
Question: Graph shows a hypothetical speed distribution for a sample of \(N\) gas particle (for \[V > {V_0}\]...
Graph shows a hypothetical speed distribution for a sample of N gas particle (for V>V0;dVdN=0,dVdN is a rate of change of number of particles with change velocity.)
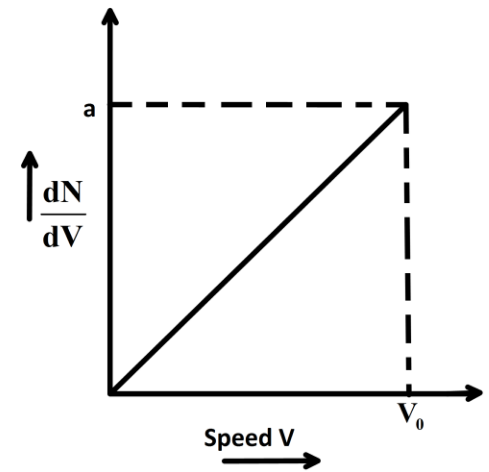
A. The value of aV0 is 2N
B. The ratio of V0Vavg is equal to 32
C. The ratio of V0Vrms is equal to 21
D. Three fourth of the total particle has a speed between 0.5V0and V0
Solution
To solve this problem find the value of the rate of change of number of particles with change in velocity. Then find the relation between the V0 and the number of particles N.
Formula used:
The equation of straight line is given by,
y=mx+c
where, m is the slope of the straight line and c is the interception on the Y-axis.
Complete step by step answer:
From the graph we can see that the slope of the curve is, V0a since the maximum value of Y-axis is a and on the X-axis it is V0 minimum value is zero. The interception on the Y-axis is zero. The Y variable is here the rate of change of number of particles with change in velocity dVdN and the X-axis plot is V0.
Hence, we can write the equation of the curve as, dVdN=V0aV
Now, we can rewrite this as, dN=V0aVdV
Integrating over the limit for N 0→N and for V 0→V0 we have,
0∫NdN=V0a0∫V0VdV
Putting the value of the limit we have,
N=V0a2V02
Up on simplifying we have, N=2aV0
So, we can see, 2N=aV0
Hence, the value of aV0 is 2N
Hence, option A is the correct answer.
Note: The r.m.s value of V is Vrms=N10∫V0V2dN which is equal to the 4NaV03 and the average value of V is Vavg=N10∫V0VdN which is equal to the value 3NaV02. Hence, all the options except option (A) are incorrect. You can perform the integration by yourself to check the answers for further insight.
