Question
Question: Glycol on heating with \({{P}}{{{I}}_3}\) mainly gives: A. ethylene B. ethylene iodide C. ethy...
Glycol on heating with PI3 mainly gives:
A. ethylene
B. ethylene iodide
C. ethyl iodide
D. ethane
Solution
Alcohols are the organic compounds having hydroxyl functional groups. Iodoform test is used to check the presence of carbonyl compounds or certain alcohols in an unknown compound. Iodoform test is used only for secondary alcohols with a methyl group in alpha position and ethanol.
Complete step by step answer:
Glycol has a chemical formula C2H4O. Its structure is given below:
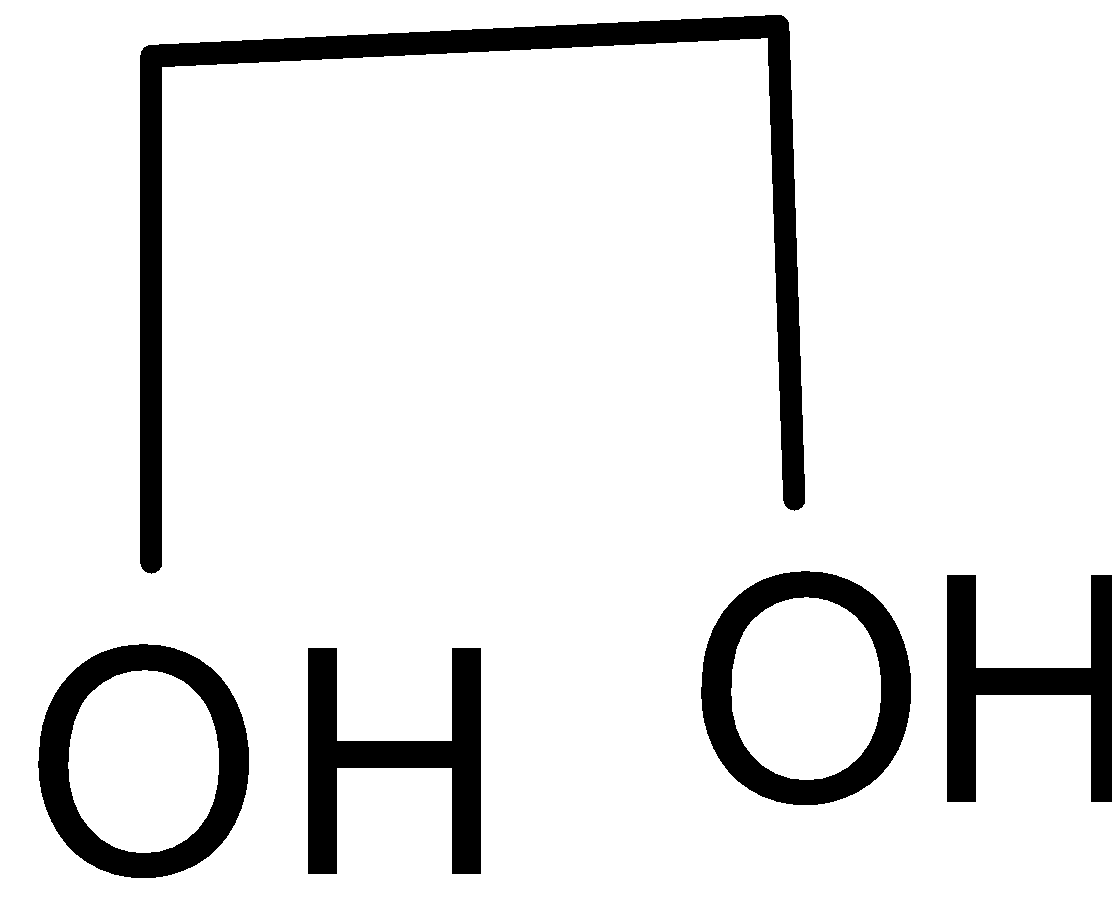
It has two hydroxyl groups, i.e. it is an alcohol. Haloform reaction is a chemical reaction where a haloform is produced by the exhaustive halogenation of aldehyde or ketone or certain alcohol in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce haloform. In iodoform test, triiodo methane is formed as a product.
Initially the alcohols are converted to aldehyde, then it is iodinated forming a yellow precipitate. Tertiary alcohols cannot be oxidized because it does not have any hydrogen atom attached to the carbon. Moreover only ethanol gives positive results for iodoform test among primary alcohols. Secondary alcohols can be oxidized to ketones, thereby giving results for iodoform test. Simple secondary alcohols with hydroxyl groups in the second carbon give positive results for iodoform test.
When glycol is heated with phosphorus triiodide, it forms ethylene diiodide initially. Then it loses its iodine and forms ethylene molecules.
Hence the correct option is A.
Note:
When ethylene glycol is reacted with PCl3, it forms ethylene dichloride. But this does not happen when it is heated with PI3. This is because ethylene diiodide is an unstable compound and it is also sterically hindered. Thus it breaks down into ethylene and iodide molecules when heated.
