Question
Question: Glucose on oxidation gives the acid containing the C-chiral atoms equal to: (A) 2 (B) 3 (C) 4 ...
Glucose on oxidation gives the acid containing the C-chiral atoms equal to:
(A) 2
(B) 3
(C) 4
(D) 5
Solution
Chiral carbon is the carbon which is asymmetric. i.e. It does not have any element of symmetry in it. Glucose gives gluconic acid upon its oxidation reaction with reagent like bromine water.
Complete Step-by-Step Solution:
We will see that product of the oxidation of glucose. Then, we will find the number of chiral carbon atoms present in the compound.
- Chiral carbon is the carbon which is asymmetric. i.e. It does not have any element of symmetry in it. We know that the valency of carbon is 4. So, it forms four bonds. Now, if all four substituent groups are different on single carbon, then the carbon is said to be chiral.
- The oxidation of glucose is generally done with Bromine water. Glucose on oxidation gives gluconic acid. Here, the aldehyde functional group of glucose gets converted to the carboxylic acid group. The reaction can be given by
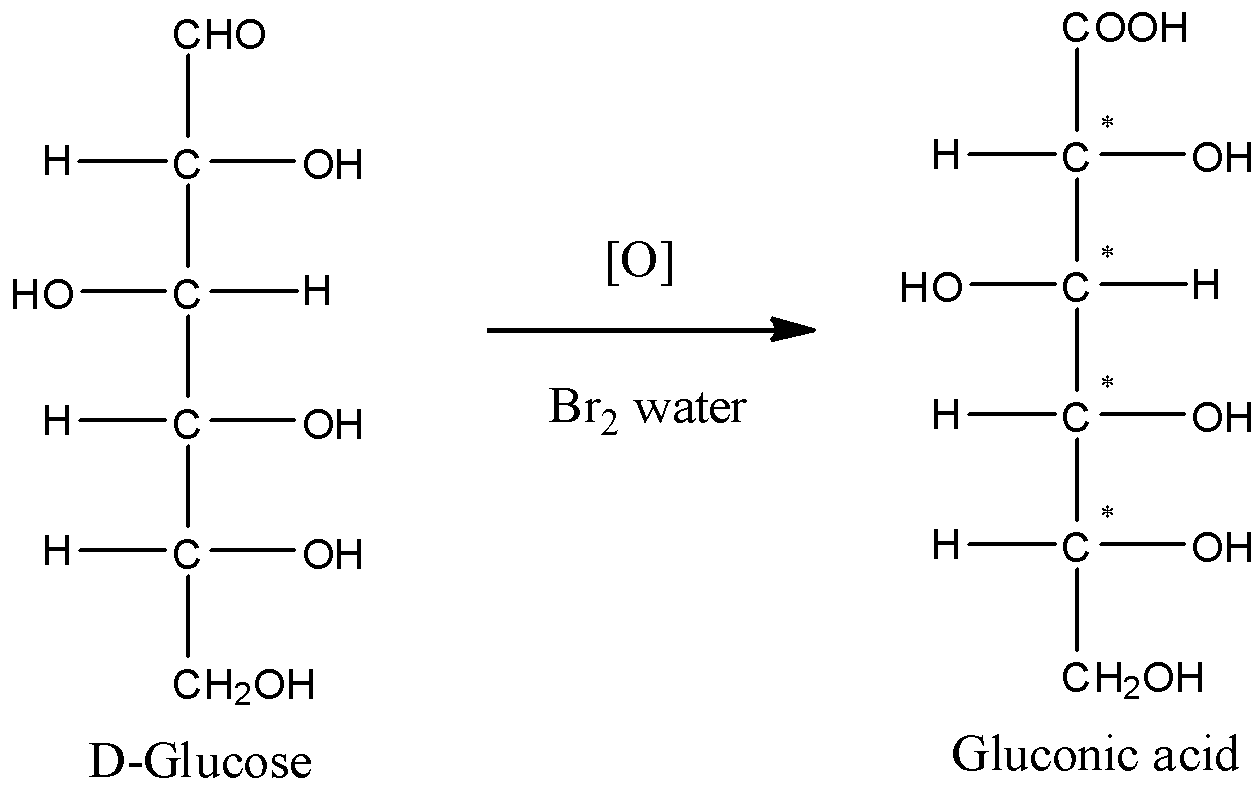
- Now, take a look at all the carbon atoms of gluconic acid. The first carbon atom forms a double bond with a single oxygen atom. So, it cannot be chiral carbon.
- The carbon-2 has one –OH group and one –H atom. The other two substituent groups are also different. So, carbon-2 is a chiral carbon.
- Carbon with numbers 3,4 and 5 are also chiral. This is because all the four substituent groups are different.
- Thus, we can conclude that there are a total of 4 chiral carbons present in the gluconic acid molecule.
So, the correct answer is (C).
Note: Note that only the aldehyde functional group of glucose gets oxidized to the carboxylic acid functional group. The hydroxyl group does not get oxidized in presence of oxidizing agents like bromine water.
