Question
Question: Glucose is an example of (A) Aldohexose (B) Ketohexose (C) Aldopentose (D) ketopentose...
Glucose is an example of
(A) Aldohexose
(B) Ketohexose
(C) Aldopentose
(D) ketopentose
Solution
Glucose consists of 6 carbon atoms and it also has an aldehyde group. The molecular formula of glucose isC6H12O6. Glucose is an organic compound.
Complete step by step solution:
We have to identify that glucose is an example of which of the given options. Glucose is an organic compound and a simple sugar. The general chemical formula for glucose is C6H12O6.The structure of D and L form of glucose is mentioned below: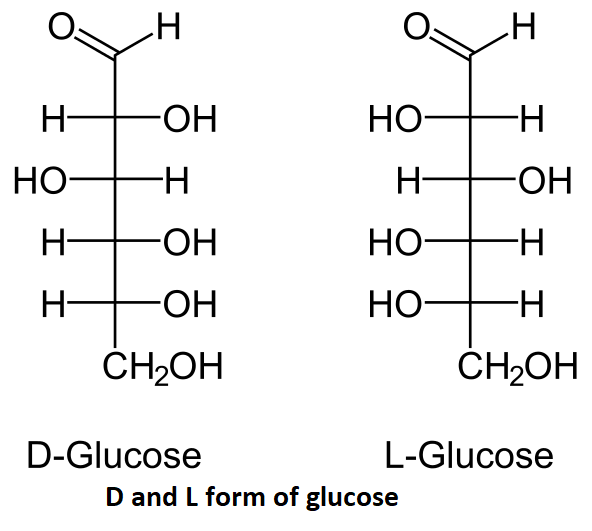
D-glucose stands for dextrorotatory glucose and L-glucose stands for levorotatory glucose, these two are the stereoisomers of glucose. From the above structure we can see that glucose contains 6 carbon atoms and it also contains 12 hydrogen atoms and 6 oxygen atoms. There is an aldehyde group present at the 6 carbon.
Aldohexose- it is a monosaccharide which contains both an aldehyde group and a six carbon chain. The aldehyde is known as aldose and the 6 carbon chain is known as hexose.
Ketohexose-it is also a monosaccharide but it has a keto group in its structure and also a carbon chain of 6 carbons.
As we already know that glucose contains 6 carbon atoms and an aldehyde group it is an Aldohexose.
Hence the correct answer is option (A) i.e. Glucose is an example of Aldohexose
Note: While solving such types of questions it is important to have prior knowledge about the structure of glucose. Glucose is also a monosaccharide which means that it is a simple reducing sugar, it is the most basic unit of carbohydrates.
