Question
Question: Glucose does not react with: A. \({Br}_2/H_2O\) B. \(N{H_2}OH\) C. \(NaHS{O_3}\) D. \(HI\)...
Glucose does not react with:
A. Br2/H2O
B. NH2OH
C. NaHSO3
D. HI
Solution
We know that the chemical reactions of a compound can be used to deduce the structure of the compound and vice versa as both are interrelated. Glucose is one of the six carbon-containing hexopyranose. It is also known that there is no free −CHO group present in the cyclic structure of glucose.
Complete answer
Glucose is a very significant bio-molecule as it is a main constituent of sweet products such as fruits, honey or sugar. It also acts as a monomer for many other carbohydrates including starch and cellulose. It has a chemical formula C6H12O6. Now, let’s have a look at its structure which can be shown as below:
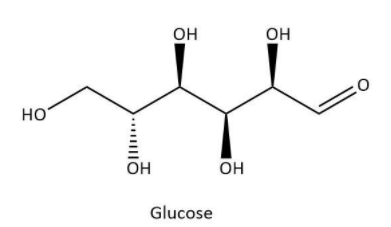
As we can see that it contains six carbons and an aldehyde group which gives it the name, aldohexose.
Now, we will discuss the reactions of glucose that results from its structure.
1. Reaction with HI: Glucose upon heating with HI gives hexane. The reaction can be written as follows:
HOCH2−(CHOH)4−CHO⟶HI,DeltaCH3(CH2)4CH3
This reaction provides evidence of six carbons being in a straight chain as the product is n-hexane.
2. Reaction with NH2OH: Glucose gives an oxime after reacting with NH2OH. The reaction can be written as follows:
HOCH2−(CHOH)4−CHO+NH2OH→HOCH2−(CHOH)4−HC=N−OH
Formation of oxime is a characteristic reaction of the carbonyl group. So, we can say that this reaction can help us in confirming the presence of >C=O group in glucose.
3. Reaction with Br2/H2O: Glucose gives carboxylic acid after reacting withBr2/H2O as bromine water acts a mild oxidizing agent and the aldehyde group gets oxidized further to the carboxylic group. The reaction can be written as follows:
HOCH2−(CHOH)4−CHO+Br2/H2O⟶HOCH2−(CHOH)4−COOH
This reaction helps us to deduce that the carbonyl group present in glucose is aldehyde.
From the above discussion, we can see that glucose reacts with three of the given reagents, Br2/H2O, NH2OH and HI.
**Hence, the reagent that it doesn’t react with is NaHSO3 making option C to be the correct one.
Note: **
We have to carefully choose the reagents because every compound shows characteristic reactions. Glucose consists of an aldehyde group. However, it does not undergo reaction with sodium hydrogen sulphite in order to form bisulphite addition products. This is due to the fact that this reaction occurs in the presence of a free aldehyde group, but there is no free −CHO group present in the structure of glucose. Therefore, we can say that sodium hydrogen sulphite is not able to cleave the cyclic ring (δ−oxidering) in glucose.
