Question
Question: Globular proteins are present in : (A) blood (B) keratin (C) muscles (D) none of these...
Globular proteins are present in :
(A) blood
(B) keratin
(C) muscles
(D) none of these
Solution
Packing efficiency can be defined as the percentage of space obtained by constituent particles that are packed within the lattice.
In a body centered cubic unit cell, one atom is located at the body center apart from corners of the cube.
Complete step by step answer:
Step by step solution:
In a body-centered cubic unit cell atoms are present at the corners of the unit cell and one atom occupies the center of the unit cell.
Packing Efficiency =TotalvolumeofunitcellVolumeoccupiedbytheatomsinaunitcell×100
The relationship between the radius r and the edge length of a unit cell is given as
a=34r
To find the volume of a unit cell we increase edge length by three-time
a3=(34r)3=3364r3
The number of atoms per unit cell in a body-centered cell is 2.
The volume of a sphere is =34πr3
Volume of each unit cell =2×34πr3=38πr3
Substituting the values in the above equation
Packing Efficiency =3364r338πr3×100=68.04
The packing efficiency of Body-Centered Cubic lattice is equal to 68.04%.
In a body-centered cubic structure, the space occupied is about the concept: Packing efficiency – Efficiency of packing in body-centered cubic structures.
So, the correct answer is Option A.
Note: Alternative method: There is an alternate method to find the packing efficiency of Body-Centered Cubic Lattice as follows:
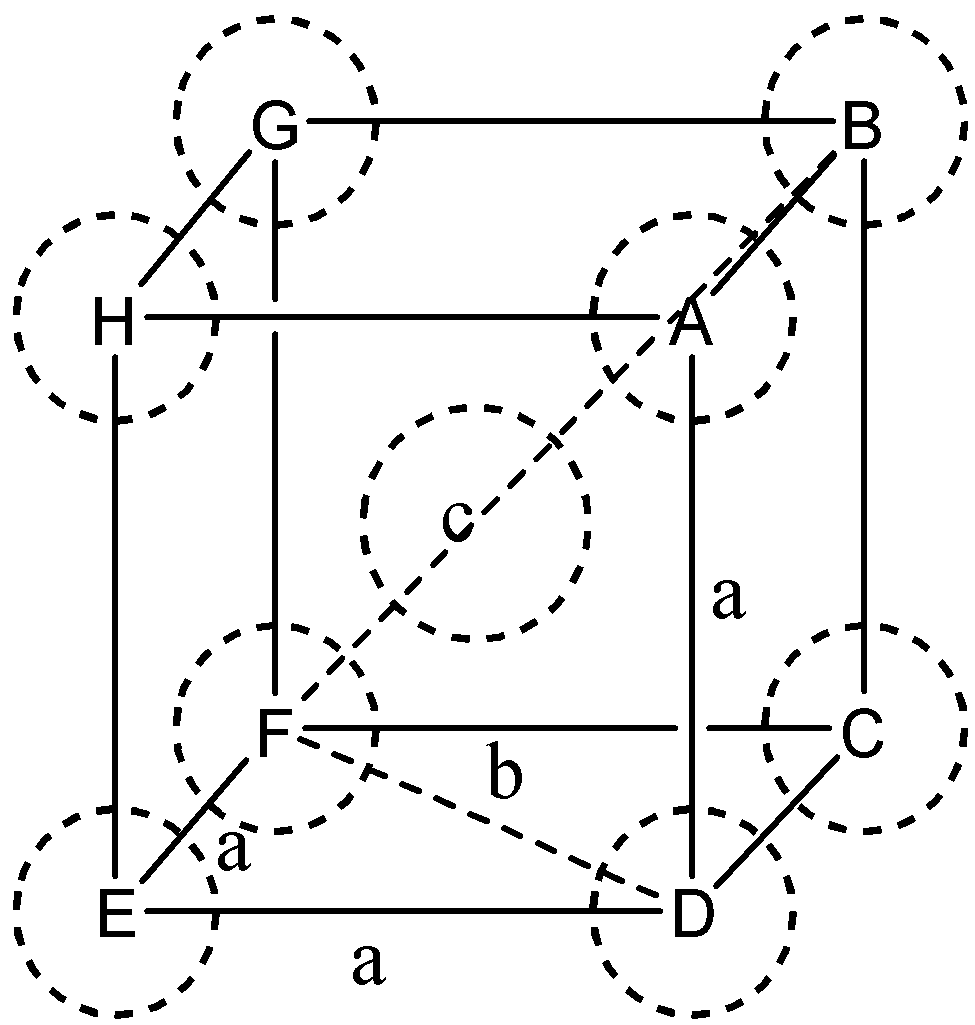
In the body-centered cubic unit cell
⇒ In ΔEFD
⇒ Let DF=b
and we know ED=EF=a (edge length)
Now,
b2=a2+a2=2a2
In ΔAFD
Let, AF=c
We know thatFD=b and AD=a (edge length)
Now,
c2=a2+b2=a2+2a2=2a2
Or c=3a
We know that c is body diagonal. As the sphere at the center touches the sphere at the corner. Therefore body diagonal c=4
⇒3a=4r
⇒r=(43)a
⇒a=34r
Volume of the unit cell a3=(34r)3=3364r3
No. of spheres in bcc =2
Volume of 2 spheres =2×3πr34
Packing.Efficiency=TotalvolumeoftheunitcellVolumeoccupiedbytwospheresintheunitcell×100%
=[(34)r]32×(34)πr3×100%
=(33)64r3(38)πr3×100%
=68%
The packing efficiency for body centered cubic lattice is 68%
