Question
Question: Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in...
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
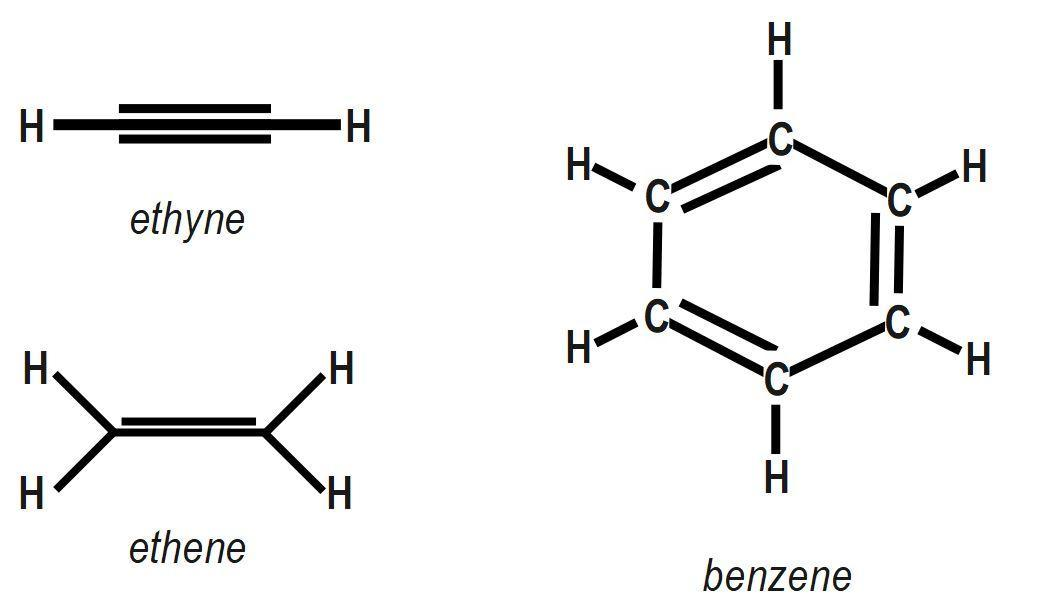
(A) Ethyne.
(B) Ethene.
(C) Benzene.
Solution
Hint : We know that the covalent bonding is seen when atoms share pairs with electrons. Atoms will covalently bond with other atoms to gain greater stability, which is gained through forming a full shell of electrons. Through exchanging most (valence) electrons out there, atoms will fill up their outer shell of electrons and gain stability.
Complete Step By Step Answer:
Covalent bonding is seen when atoms share pairs with electrons. Atoms will covalently bond with other atoms to gain greater stability, which is gained through forming a full shell of electrons. Through exchanging most (valence) electrons out there, atoms will fill up their outer shell of electrons and gain stability. If there are two chlorine atoms, with seven valence electrons each for chlorine. Thus, each of these chlorine atoms only needs one more electron to complete the valency of its outer shell. These atoms bind together by sharing two electrons and forming one bond. The negative electrons are driven into each atom's positively charged nucleus, thereby holding the atoms together. Based on the number of pairs of electrons shared between atoms, there are three types of bond. They are:
Single bond: A single bond is formed when one pair of electrons is shared between two atoms. This bond type is relatively weak and has a smaller electron density than a double bond and a triple bond, but is the most stable because it has a lower reactivity level. It means that the loss of electrons to atoms is less susceptible.
Double bond: A Double bond is formed by the two atoms sharing two pairs of electrons. This type of bond is stronger than a single bond but less stable because of its greater reactivity than a single bond. Double lines (=) represents a double bond between two atoms (i.e., involving two electron pairs.
Triple bond: A Triple Bond is formed when a molecule shares three pairs of electrons between two atoms. It is the least stable of the three general types of covalent bonds, because losing an electron is very vulnerable. Triple lines (≡) indicates a triple bond.
| Compound/Bond type | Single Bond | Double Bond | Triple Bond |
|---|---|---|---|
| Ethyne | Two | None | One |
| Ethene | Four | One | None |
| Benzene | Nine | Three | None |
Note :
Remember that when pairs of electrons are shared between the atoms it results in the formation of covalent bonds. The octet rule explains that atoms of main group elements seem to bind So that all of the atoms have eight electrons in their valence shells, and attain the same noble gas electronic configuration. If one pair of electrons are shared between atoms, then a single bond is formed.
