Question
Question: Given X is an optically active alkane having lowest molecular mass, predict the structure of the maj...
Given X is an optically active alkane having lowest molecular mass, predict the structure of the major product obtained on monochlorination of X
A.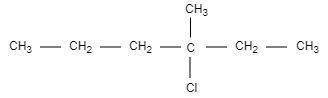
B.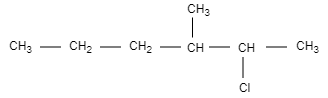
C.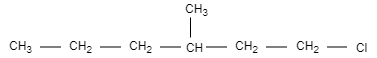
D.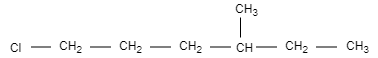
Solution
-Chiral compounds are the compounds that cannot be superimposed on its mirror image by the combination of rotation. Chiral compounds exist in two isomers that show mirror images of each other. -These are also enantiomers.
Complete step by step answer:
A chiral molecule consists of a chiral center. When this center coincides with another atom, that substance is said to have point chirality. A compound is said to be chiral when there is no plane of symmetry and center of symmetry. If it does not have either of these, then it is said to be chiral. A chiral compound is said to be an optically active compound that shows non superimposable mirror images on itself.
The optically active alkane having lowest molecular mass is 3− methyl-hexane.
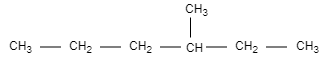
Let us discuss the given options in this question,
A.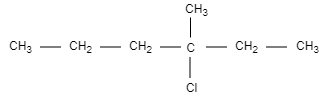
In this structural formula, we can see that carbon is attached to four different groups and hence, we can say that structure in option (A) is chiral and optically active.
B.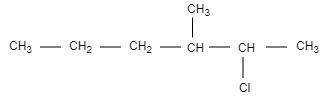
In this option, the structure is not chiral and optically inactive as there are no four different groups attached.
C.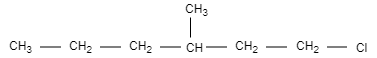
In this option, the structure is not chiral and optically inactive as there are no four different groups attached.
D.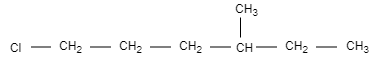
In this option, the structure is not chiral and optically inactive as there are no four different groups attached.
When 3− methyl-hexane is monochlorinated, it results in the formation of 3− chloro −3− methyl-hexane.
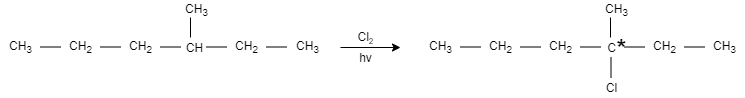
The product formed in the above reaction is chiral and optically active.
Therefore, the correct option is (A).
Note: Chiral compounds are the compounds that do not show superimposable mirror images, whereas achiral compounds are the compounds that show superimposable mirror images of each other.
-Chiral compounds do not show planes of symmetry or center of symmetry, whereas achiral compounds show planes of symmetry or center of symmetry.
-In conclusion, the X compound is 3− methyl-hexane, which is monochlorinated to give 3− chloro −3− methyl-hexane.
