Question
Question: Given the structural formula for the following: Ethyne....
Given the structural formula for the following: Ethyne.
Solution
As it is given in the question, we can see that Ethyne has a suffix – “yne”. This is a term used in IUPAC nomenclature to indicate triple bond. Also, the word “eth” is a term which is used to describe organic compounds having two carbons. So, ethyne has two carbons and a triple bond between them. For a carbon-carbon triple bond, the hybridisation of the carbon atoms is sp. Using all this information find out the structural formula.
Complete step by step answer:
In Organic chemistry, the word “eth” means two and “yne” means triple bond. So the compound Ethyne means a compound having two carbon atoms and a triple bond. Ethyne is the systematic IUPAC name of the chemical compound having a formula of C2H2 (consisting of two carbon atoms and two hydrogen atoms). The compound is commonly known as Acetylene. It is a hydrocarbon and the simplest alkyne.
Ethyne has a triple bond. A triple bond is usually formed by one sigma bond and two pi bonds. Pi bonds are formed by the lateral overlapping of atomic orbitals which are not hybridised. The hybridised atomic orbitals overlap with each other forming a sigma bond.
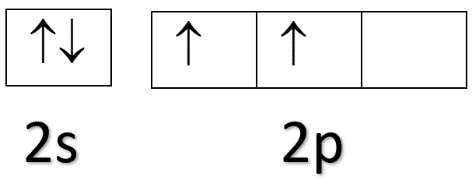
Now, the electronic configuration of carbon is 1s22s22p2.
From this, we can see that carbon has 2 electrons in 2p orbital at the ground state. In the excited state during the bond formation, one electron will move from 2s orbital to one of the vacant space in 2p orbital and the electronic configuration will be 1s22s12p3.
This is represented below:
Ground state electronic configuration of carbon:
Excited-state electronic configuration of carbon:
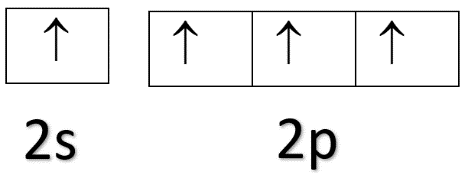
Now, since carbon forms two pi bonds, two 2p electrons will take part in the bond formation which will leave us with one 2s electron and one 2p electron. These orbitals will undergo hybridisation forming two sp hybrid orbitals. One will form a sigma bond with hydrogen while the other will form a sigma bond with another carbon atom in ethyne.
Since the carbon atoms in ethyne have an sp hybridisation, the structure of ethyne will be linear. The structure of ethyne is given below.
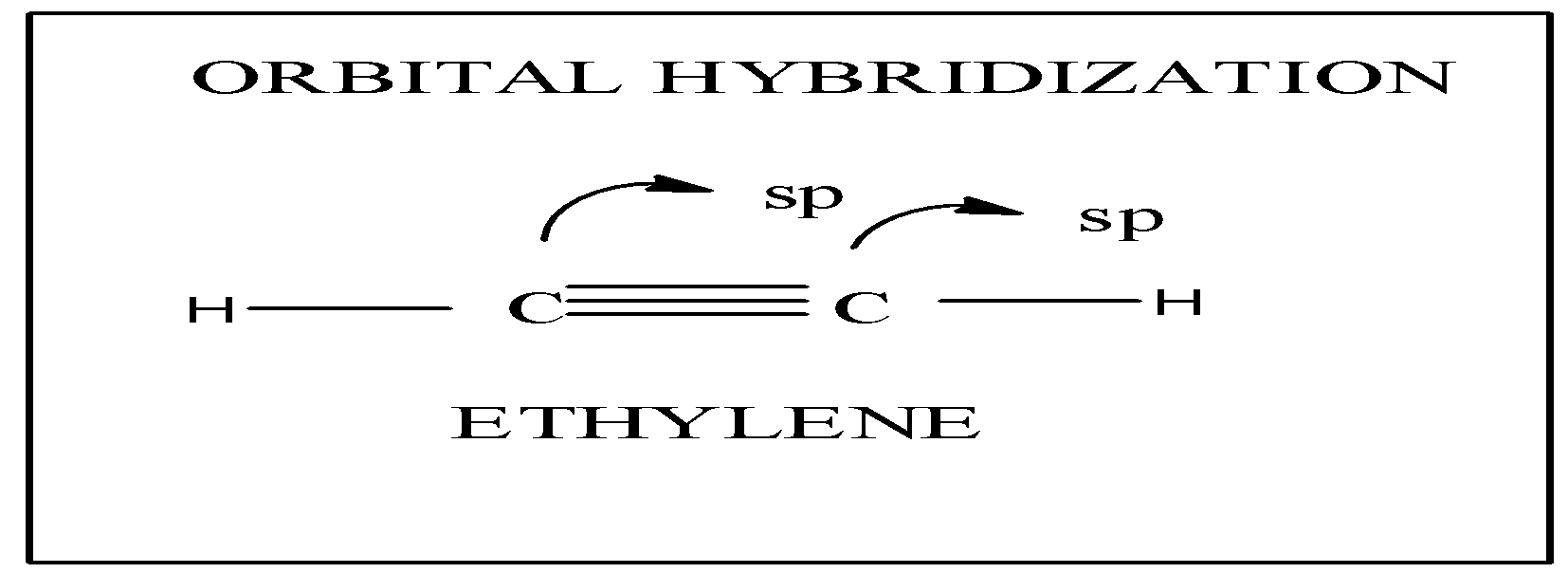
The bond angle between hydrogen & carbon and carbon and hydrogen 180∘.
Note: Always remember that in case of alkanes the first member of its homologous series is methane as “meth” in organic chemistry means one carbon atom. But this is not true in case of alkenes and alkynes as they contain carbon-carbon double and triple bonds and for this minimum of two carbon atoms are required. So, the first members of alkene and alkyne homologous series will be ethane and ethyne respectively.
Also for the calculation of orbital hybridization, only count the sigma (σ) bonds. Don’t include the pi (π)bonds as they don’t take part in the hybridisation.
