Question
Question: Given the following sequence of reaction: \(C{H_3}C{H_2}I\xrightarrow{{NaCN}}A\xrightarrow[{Partia...
Given the following sequence of reaction:
CH3CH2INaCNAOH−Partial hydrolysisBBr2/NaOHC
The major product C is:
(a)CH3CH2NH2
(b)CH3−CH2−COONH4
(c)CH3CH2−COONH4
(d)CH3−CH2CO−NBr2
Solution
To start this question, you can recall the concept of nucleophilic substitution reactions. nucleophilic substitution reactions are those reactions in which an electron pair donor (i.e. a nucleophile say ‘Y’:) reacts with an electron pair acceptor (i.e. a substrate, say ‘R-X’) and which substitutes for the ‘X’ group (i.e. a leaving group). Let us look at the following generalized equation for nucleophilic substitution:
Y:−+R−X→Y−R+:X−
Here, R can be an alkyl or an aryl group.
Complete step by step answer:
We know that Sodium cyanide is used to substitute cyanide ions into the given which it reacts with. It does a nucleophilic substitution reaction. Bromine in presence of sodium hydroxide is used in bromamide degradation synthesis.
Now, let us solve the given question in a step-wise manner:
CH3CH2I in the presence of NaCN would lead to the formation of CH3CH2CN via nucleophilic substitution reaction. The reaction would be as follows:
CH3CH2INaCNCH3CH2CN
Thus, the product A is CH3CH2CN
Now, we know that hydrolysis of nitriles lead to the production of amide (ethanamide in the present case) as depicted below:
CH3CH2CNOH−Partial hydrolysisCH3CH2CONH2
Thus, the product B is CH3CH2CONH2
Now, ethanamide in the presence of Br2/NaOHwill lead to the formation of primary amine (with one less carbon atom) via hofmann rearrangement. The reaction is depicted below:
CH3CH2CONH2Br2/NaOHCH3CH2NH2
The mechanism of this reaction is also demonstrated below:
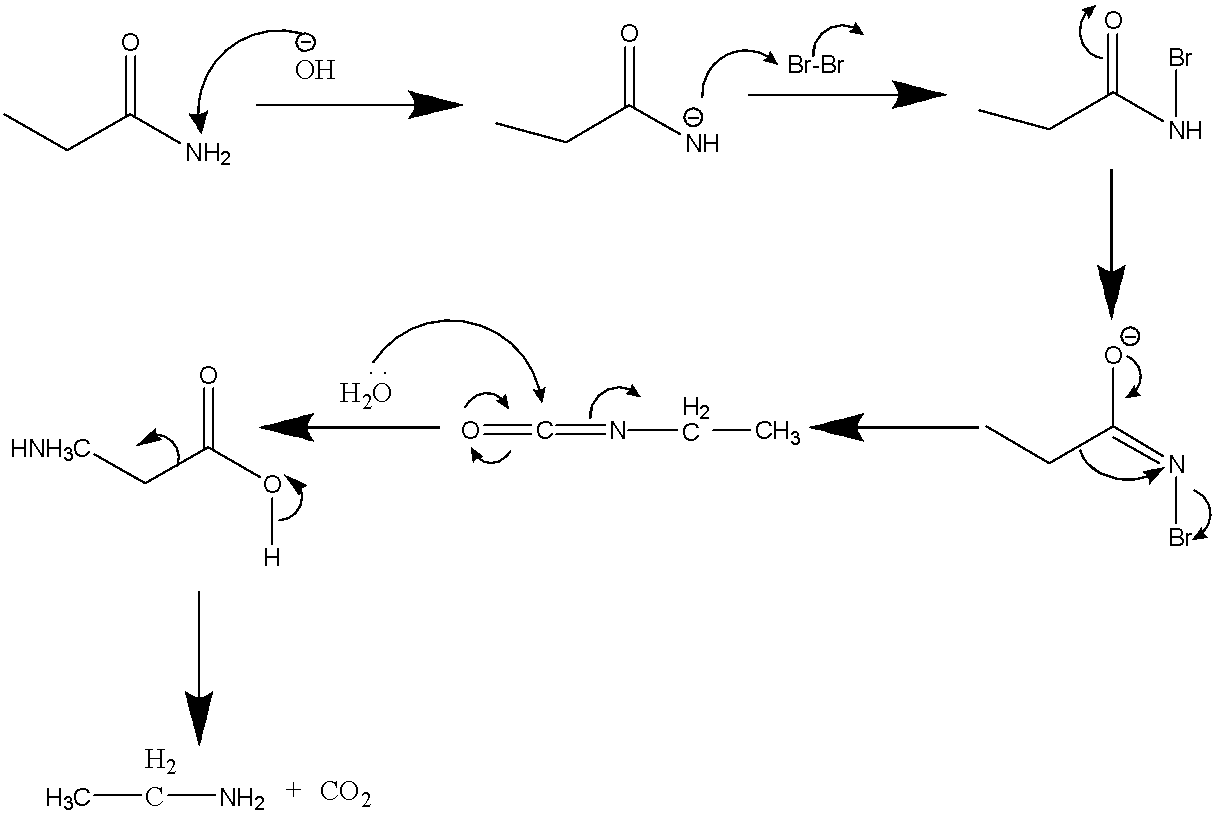
Therefore the major product C is ethyl amine that is CH3CH2NH2
The following reaction sequence would be followed:
CH3CH2INaCNCH3CH2CNOH−Partial hydrolysisCH3CONH2Br2/NaOHCH3NH2
So, the correct answer is Option a.
Note: Hofmann bromamide degradation is an important reaction for the preparation of amines. It is also used as a test for amines.
