Question
Question: Given,\[{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] \[\underri...
Given,K2Cr2O7 Δ(P) ; product (P) is :
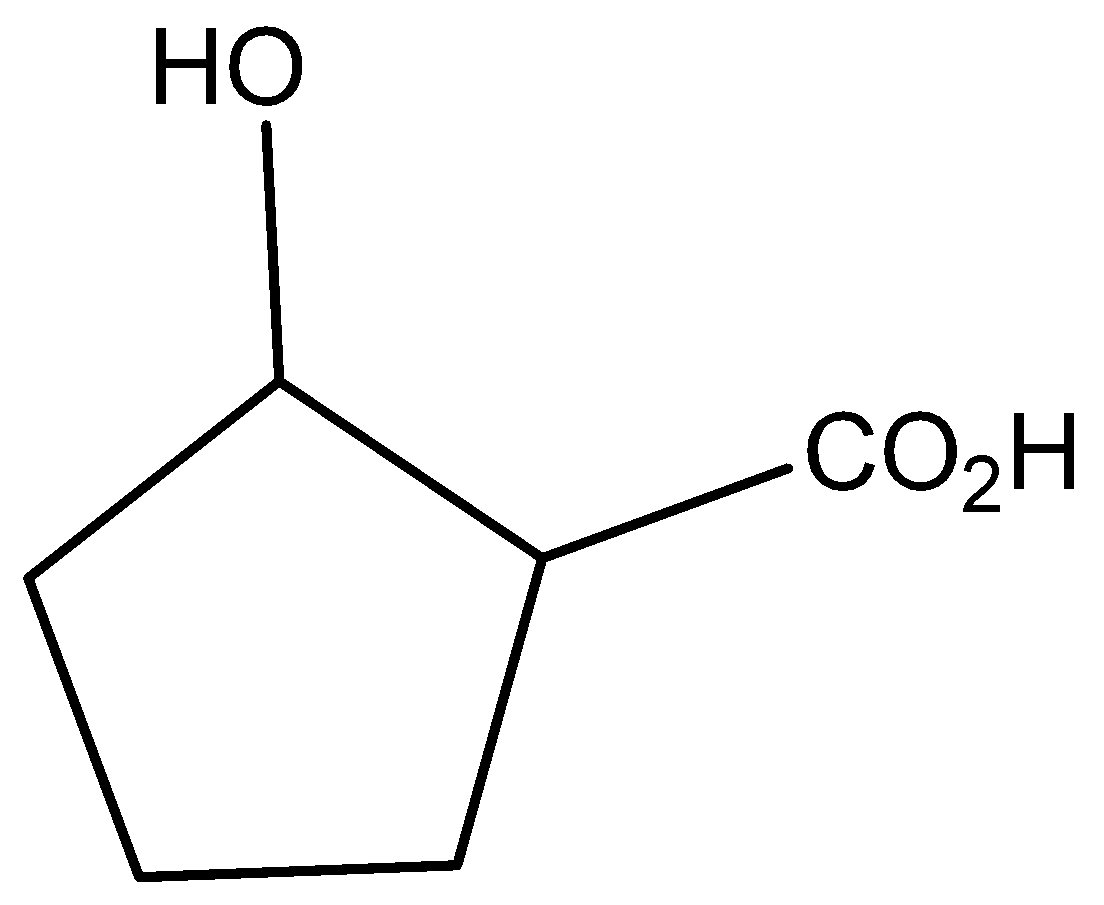
A) 
B) 
C) 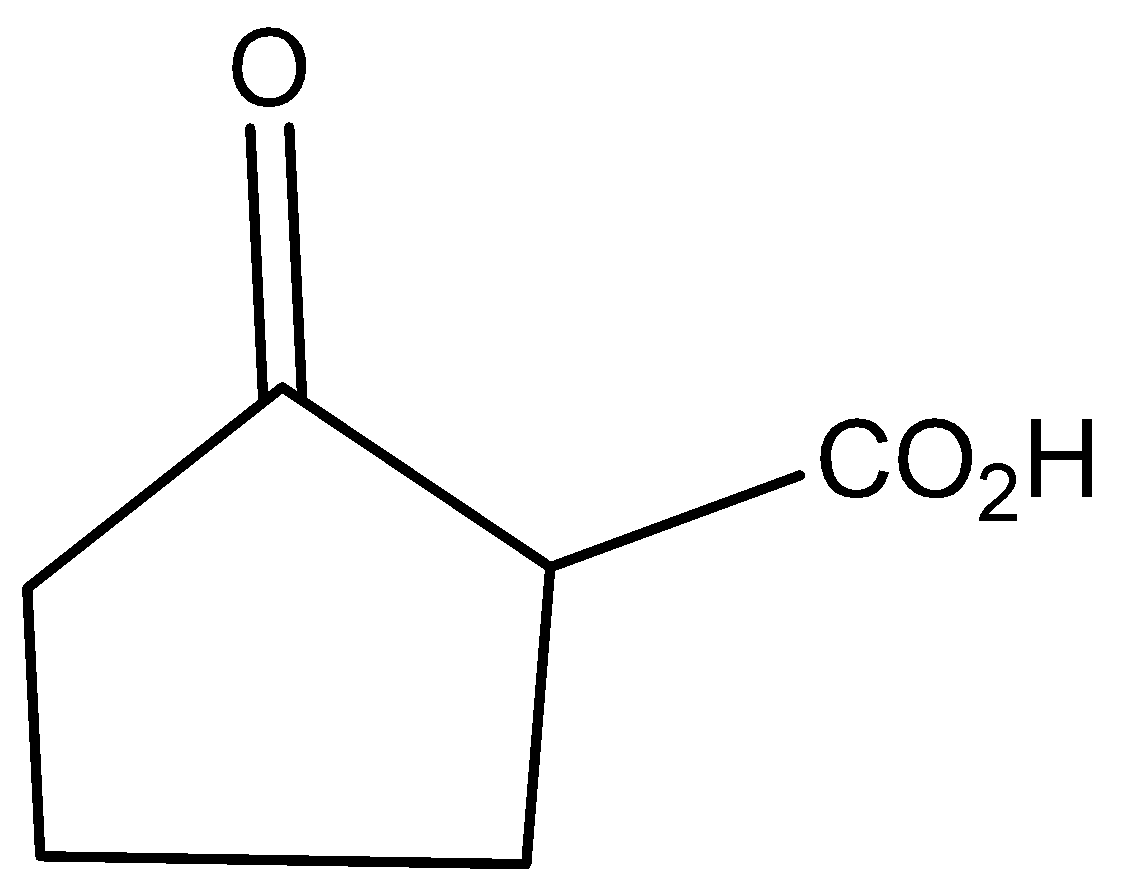
D) 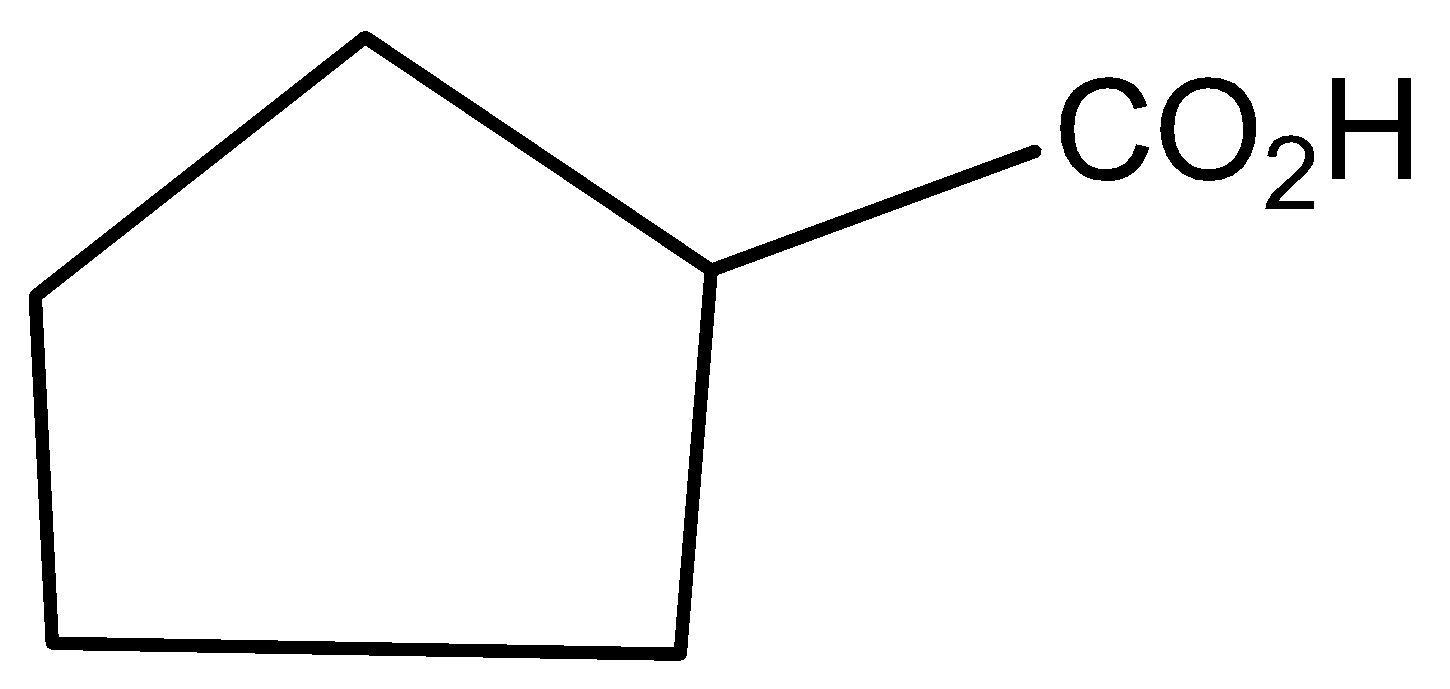
Solution
In chemistry two reactions are main in organic concept. The two reactions are oxidation and reduction reaction. The oxidation reaction is nothing but in chemical reaction from reactant to product the addition of oxygen or removal of hydrogen or gain of electron in the product. The reduction reaction is nothing but in chemical reaction from reactant to produce the addition of hydrogen or removal of oxygen or loss of electrons. The oxidation or reduction reaction plays a key role for converting one functional group to another functional group in the organic chemical reaction.
Complete answer:
The given data is
The molecular formula of potassium dichromate is K2Cr2O7.
The molecular formula of 2-hydroxycyclo pentatonic acid is
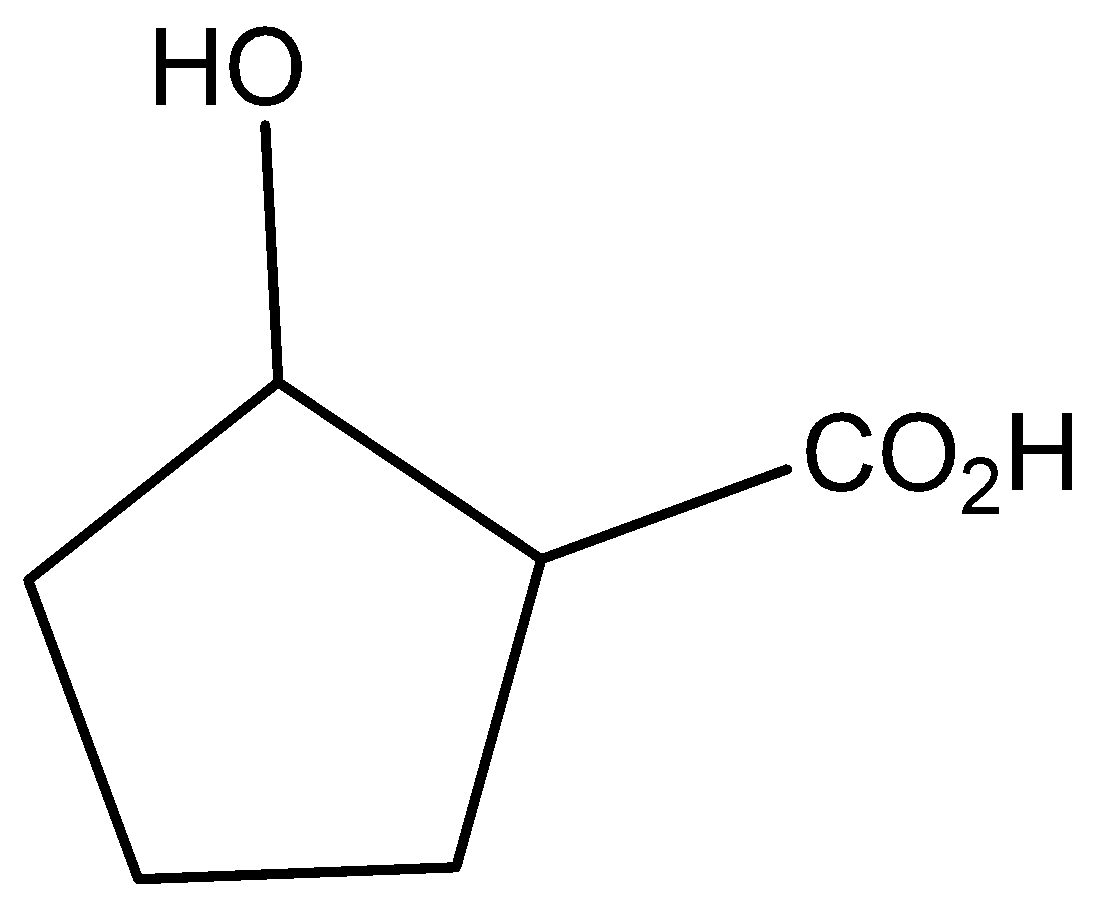
The molecular formula of cyclopentane is
The molecular formula of cyclopentane is
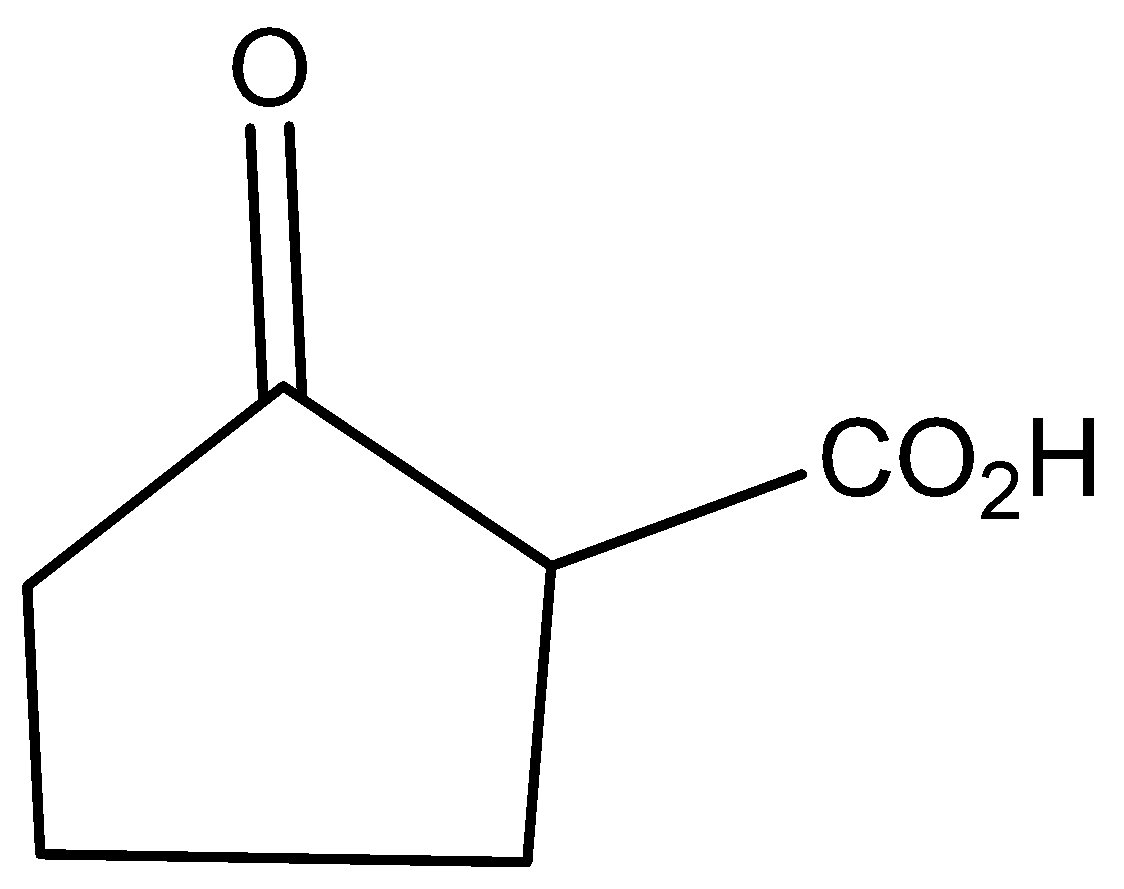
The molecular formula of 2-oxo cyclo pentatonic acid is
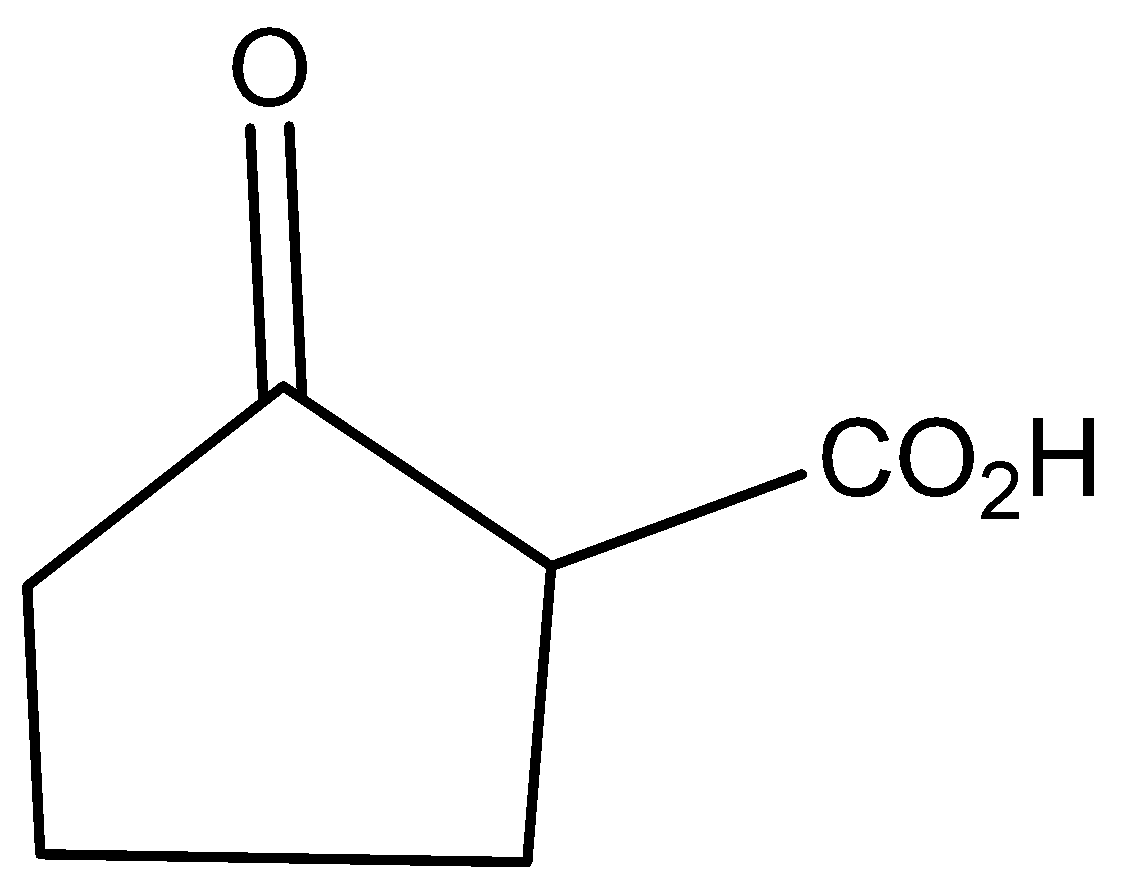
The molecular formula of cyclo pentatonic acid is
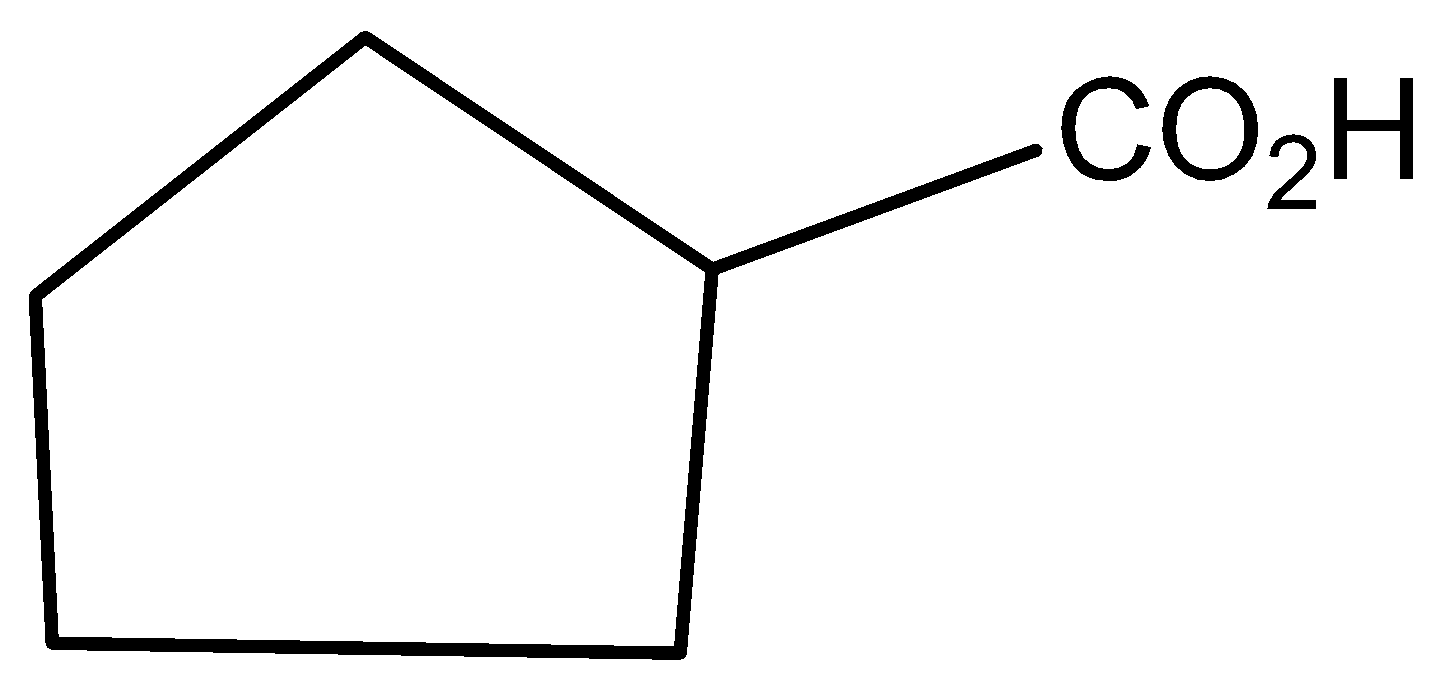
Potassium dichromate is reacted with 2-hydroxycyclo pentatonic acid to give the product of 2-oxo cyclo pentatonic acid.
The reaction for above discussion is even below,
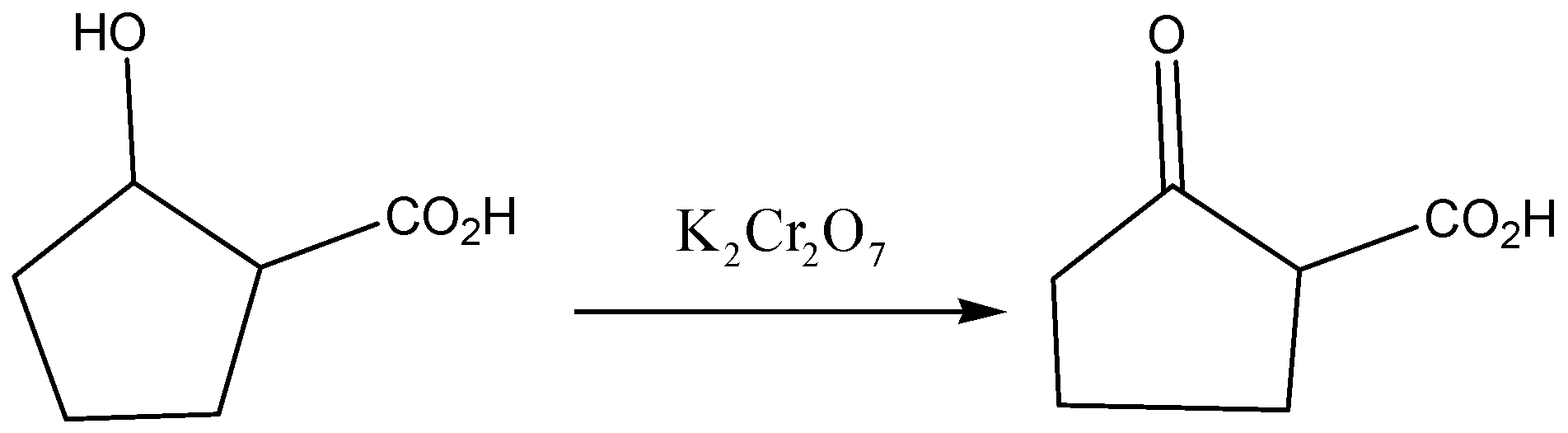
According to the above discussion, we conclude Potassium dichromate is reacting with 2-hydroxycyclo pentatonic acid to give the product of 2-oxo cyclo pentatonic acid.
Hence, option C is the correct answer.
Note:
We must have to know that oxidation and reduction are reversible reactions in organic chemistry. For example, the oxidation of primary alcohol is aldehyde. At the same time, the reduction of aldehyde is primary alcohol. Hence, the oxidation of primary alcohol and reduction of aldehyde is a reversible reaction. The hydrogenation means two hydrogen atoms are added in the reactant to give product after attacking these two hydrogens. The maximum two hydrogens are attacking in the electrophilic group in the reactant by using some specific reducing reagent.
