Question
Question: Given, \[{\text{1,2 - dibormopropane}}\] on treatment with X moles of \[{\text{NaN}}{{\text{H}}_{\te...
Given, 1,2 - dibormopropane on treatment with X moles of NaNH2 following treatment with ethyl bromide gives a pentyne. The value of X is:
A) 1
B) 2
C) 3
D) 4
Solution
As we know that the mole is the one of the main units in chemistry. The moles of the molecule depend on the mass of the molecule and molecular mass of the molecule. Chemical reactions are measured by moles only. The number of equivalents of the reactant also depends on the moles of the molecule. The number of moles of the reactant and product are equal in the equilibrium reaction. Moles are defined as the given mass of the molecule is divided by the molecular mass of the molecule.
Moles=Molecular weight of the moleculeMass of the molecule
Complete answer:
We have to know that the molecule formula of 1,2 - dibormopropane is CH2(Br) - CH(Br) - CH3.
The structural formula of 1,2 - dibormopropane is
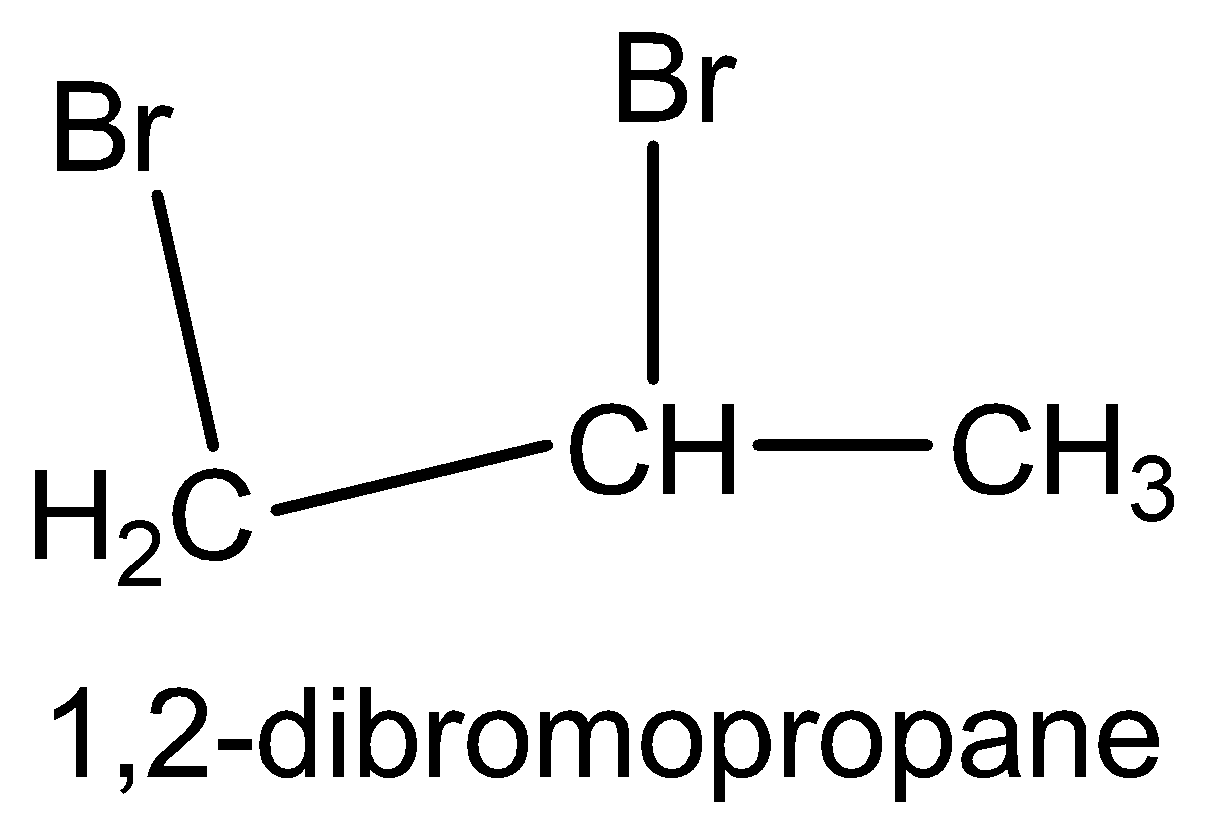
The molecule formula of ethyl bromide is CH3 - CH2Br.
The structural formula of ethyl bromide is
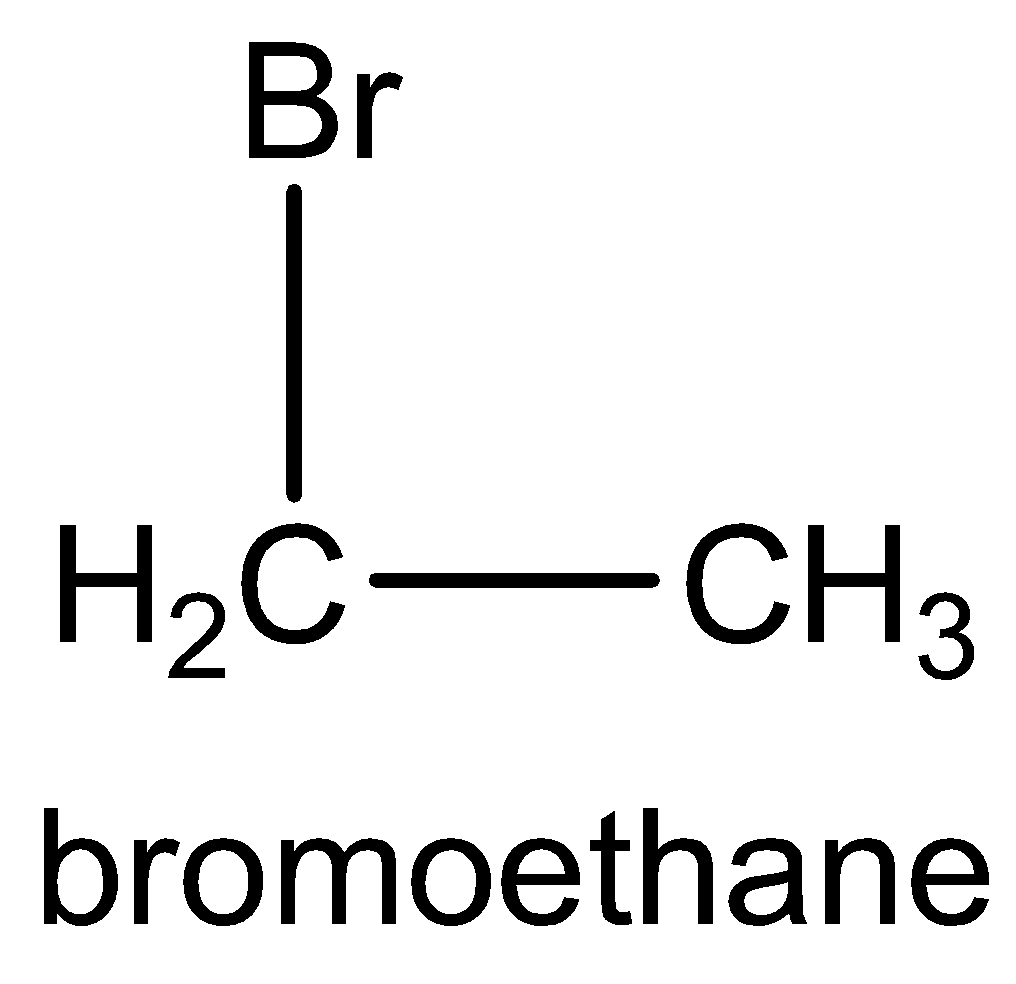
The IUPAC name of ethyl bromide is bromoethane.
The molecule formula of pentyne is CH3 - CH2 - C≡C - CH3.
The structural formula of pentyne is
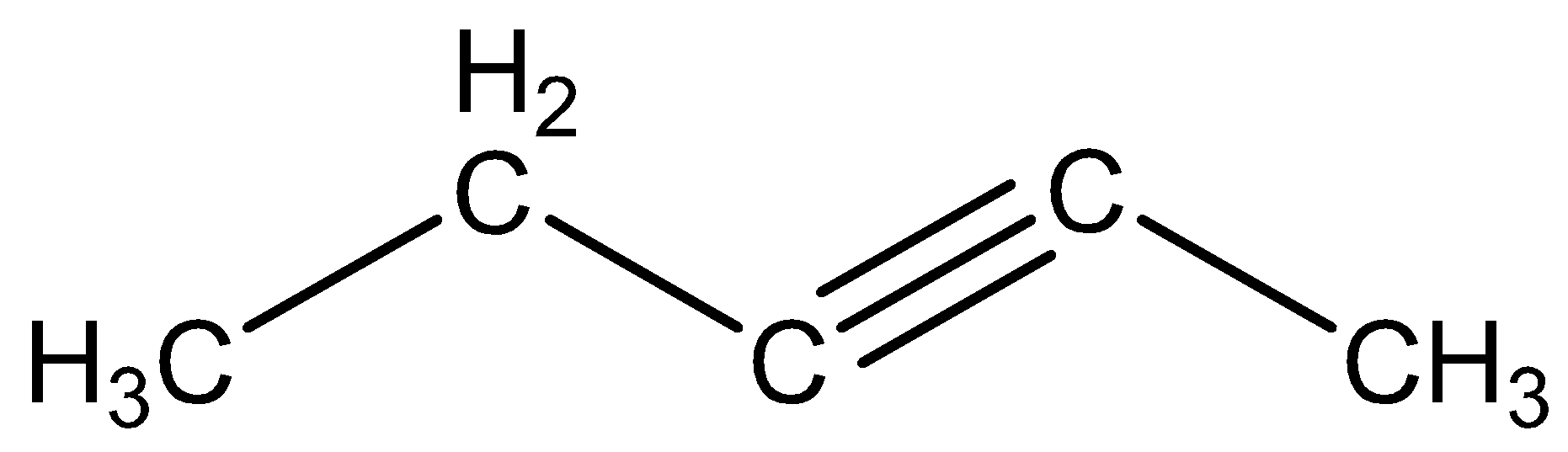
The reaction of 1,2 - dibromopropane on treatment with X moles of NaNH2 following treatment with ethyl bromide gives a pentyne is given below,
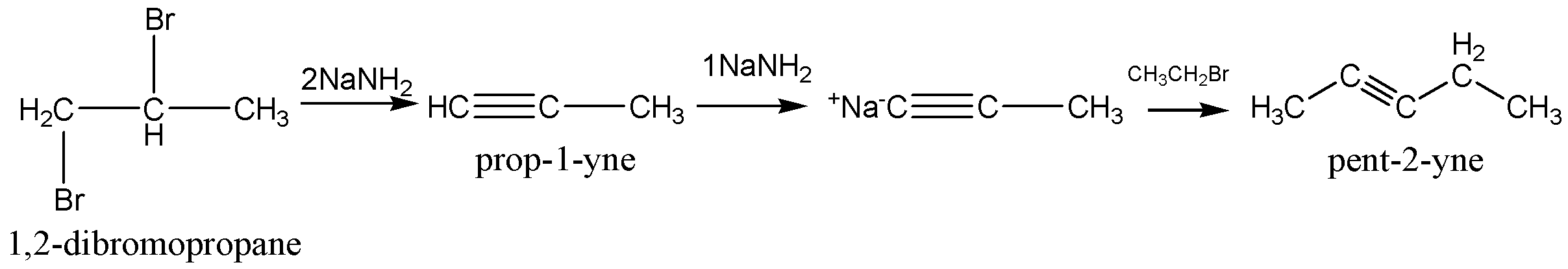
From the above reaction sodium amide three times used in the chemical reaction. So, the value of X is 3.
Hence, Option C is correct.
Note:
We also need to remember that the number of moles of the reaction if calculated, in this way we can predict the reaction is not. The number of moles are used to find the yield percentage of the reaction. The moles are also used to find the formation of side products in the chemical reaction. Carbon dioxide is one of the gases used for cooling the reaction. If one reaction, carbon dioxide evolves means that reaction is considered as an endothermic reaction.
