Question
Question: Given,\[SO_4^{2 - } \to {S_2}O_8^{2 - } + 2{e^ - }\](ox.) How it is possible as there is no change i...
Given,SO42−→S2O82−+2e−(ox.) How it is possible as there is no change in the oxidation state of S from sulphate ion to thionate i.e. +6 to +6
Solution
Check the structure of both SO42−and S2O82− to know what the change of oxidation state is. S2O82− has a peroxide linkage whereas SO42− doesn’t.
The structure of S2O82− is given below:
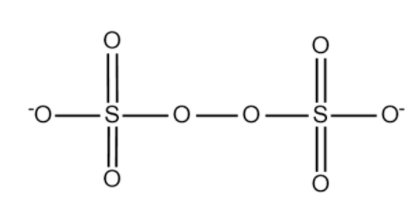
The structure of SO42− is given below:
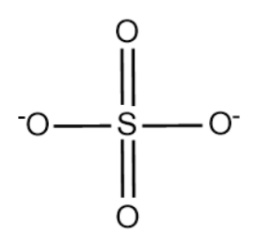
Complete answer:
Here we note that even though Sulphur doesn’t show any change in oxidation state, Oxygen does have a change. The peroxy bond in S2O82− disappears when it becomes SO42−. While counting the oxidation number of sulphur in S2O82− we consider the oxidation states of those oxygens (in peroxy bonds) to have -1 state. So from SO42−, where those 2 oxygen atoms had -2 oxidation state, we have lost 2 electrons when the peroxy bond is formed and got S2O82−. This means that there is net oxidation.
From -2 to -1 change in oxidation state means that there is net increase in oxidation state
This means oxidation
To summarise, change in oxidation is not due to the sulphur atom but due to the oxygen atom present in them. Sulphur oxidation state is +6 in both S2O82− and SO42−.
Note:
To calculate the Oxidation state of sulphur in S2O82−
Total there are 8 oxygen atoms in which 2 forms peroxy bonds. So we have 6 oxygen atoms with -2 oxidation state and 2 oxygen atoms with -1 oxidation state.
So we can say that
⇒2×x+(2×−1)+(6×−2)=−2
Where x is the oxidation state of sulphur
⇒2x−2−12=−2
⇒2x−14=−2
⇒2x=12
⇒x=6
Hence we can say that oxidation state of sulphur is +6 in S2O82−
To calculate the Oxidation state of sulphur in SO42−
Total 4 oxygen atom each having -2 oxidation number
⇒x+(4×−2)=−2
Where x is the oxidation state of sulphur
⇒x−8=−2
⇒x=6
Hence we can say that oxidation state of sulphur is +6 in SO42−
